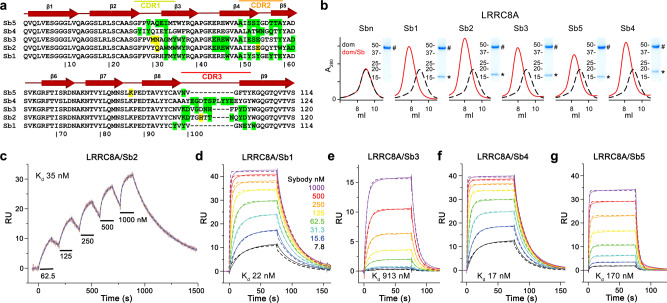
Fig. 1. Biochemical characterization of sybodies targeting the LRR domains.
a Sequence alignment of the five modulatory sybodies. Residues involved in the interaction with the primary subunit are highlighted in green, contacts to the secondary subunit in yellow. Secondary structure elements and location of CDRs are indicated above. b Sections of size-exclusion chromatograms showing the elution of the LRR domain of LRRC8A (black dashed line) and its complex with interacting sybodies (red). Sbn refers to the mixture of the LRRC8A domain with a sybody targeting a protein unrelated to the LRRC8 family. Insets show SDS-PAGE gels of the peak fractions with the LRR domain of LRRC8A (#) and co-eluted sybodies (*) indicated. Numbers refer to the molecular weight (kDa). c–g SPR experiments of sybodies binding to immobilized LRR domains of LRRC8A. c Characterization of the binding properties of sybody Sb2. Bars indicate application of the sybody at increasing concentrations (nM). d Affinity determination of sybodies Sb1, e Sb3, f Sb4, and g Sb5. d–g Individual traces show association and dissociation of sybodies at concentrations indicated in d and coded in the same color. c–g Dashed lines superimposed on the respective recordings and dissociation constants were obtained from a fit to a 1:1 binding model.