Abstract
In March 2020, the World Health Organization declared COVID-19 as the first coronavirus-initiated pandemic. COVID-19’s fast-paced global spread with a broad range of clinical manifestations compelled health regulatory organizations, public health professionals, and researchers to update their information about the disease and provide individual- and community-based guidelines, solutions, and regulations to break the disease cycle, mitigate person-to-person transmission, and reduce cross-contamination in healthcare settings. In this review, the authors provide known facts and updated information about SARS-CoV-2 virology and its new variants, transmission routes, reported clinical symptoms, epidemiology, and infection control and prevention guidelines with a focus on a hierarchy of controls in dental settings.
The first cases of coronavirus disease 2019 (COVID-19) resulting from SARS-CoV-2 infection were reported to the US Centers for Disease Control and Prevention (CDC) in December 2019.1 In March 2020, the World Health Organization (WHO) declared COVID-19 as the first coronavirus-initiated pandemic, with the virus causing drastic global, social, and economic consequences.2 At the time of this writing, 223 countries/regions have reported COVID-19 cases with the United States being among the most affected. About 148 million cases have been confirmed worldwide with 85 million recovered and 3.1 million deceased.3 In the United States, 32 million cases have tested positive for COVID-19 with 25 million cases recovered and more than 570,000 COVID-19 deaths.3,4 Given the current active cases, the mortality rate that was initially reported as 2%5 increased to about 4% in March 20206 (similar to H1N1; less than SARS-CoV; less than MERS-CoV).5 However, current mortality rates differ globally, ranging from 1.4% in India and 1.8% in the United States to 8.9% in Mexico.3 Mortality rates are higher in individuals who are elderly or have underlying medical conditions, such as hypertension, diabetes, cardiovascular diseases, and renal diseases5,7; mortality increases from 0.9% in people without comorbidities to 6% in hypertensive patients, 7.3% in diabetics, and 10.5% in people with cardiovascular disorders.7 In addition, US racial/ethnic minorities, such as non-Hispanic American Indian/Alaska Native, non-Hispanic Black/African American, and Hispanic/Latino populations, have had higher COVID-19 infection, hospitalization, and mortality rates than non-Hispanic White/Caucasian populations.8
Since COVID-19 emerged, cumulative case incidence has quickly risen globally.3,5 COVID-19’s fast-paced global spread has compelled health regulatory organizations, public health professionals, and researchers to provide individual- and community-based guidelines, solutions, and regulations to break the disease cycle, mitigate person-to-person transmission, and reduce cross-contamination in healthcare settings.
The aim of this review is to provide an overview of SARS-CoV-2 virology, transmission routes, clinical symptoms, epidemiology, and infection control and prevention guidelines by focusing on the hierarchy of controls in dental settings. Table 1 provides an overview of key points related to this review.
Table 1.
Key Points
| 1. SARS-CoV-2 interacts with the host cells’ angiotensin-converting enzyme 2 (ACE2) which is highly expressed in the heart, lungs, kidney, and gastrointestinal tract playing a key role in COVID-19 clinical manifestations. |
| 2. Mortality rates are higher in elderly and individuals with underlying medical conditions, such as hypertension, diabetes, cardiovascular diseases, and renal diseases. |
| 3. US racial/ethnic minorities, such as Black/African American, American Indian/Alaska Native, and Hispanic populations, have had higher COVID-19 infection, hospitalization, and mortality rates. |
| 4. Virus variants have raised researchers’ concerns about the potential for increased transmissibility and an impact on immunity. |
| 5. COVID-19 is mainly transmitted from infected (pre-symptomatic, asymptomatic, and symptomatic) individuals to close contacts. |
| 6. SARS-CoV-2 spreads through airborne infectious particles and droplets, environmental contacts, and cross-contamination. |
| 7. Primary infected individuals on average lead to 2 to 3 secondary infectious cases. |
| 8. Clinical symptoms depend on the body organ the virus has infected. |
Coronavirus Virology
Coronaviruses are comprised of four genera, among them betacoronaviruses, which are enveloped positive-stranded RNA viruses. The Coronavirus Study Group of the International Committee on Taxonomy of Viruses has proposed that this virus be designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).9 SARS-CoV-2 is one of seven coronavirus strains emerging since the 1960s and one of three, along with severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), resulting in severe life-threatening respiratory symptoms.10 Similar to SARS-CoV and MERS-CoV, SARS-CoV-2 has a zoonotic source, originating in bats and transmitted to another species before humans; the closest RNA sequence similarity is to two bat coronaviruses, so bats are the likely primary source.11 Whether COVID-19 virus was transmitted directly to humans from bats or through some other mechanism is unknown.12
Notable Variants
Like other viruses, SARS-CoV-2 evolves and mutates over time. Most mutations in the SARS-CoV-2 genome have no impact on viral function. Certain variants of concern (VOCs) have garnered widespread attention because of their rapid emergence within populations and potential for increased transmission and/or more severe clinical implications (including mortality).
United Kingdom (UK) variant (VOC B.1.1.7 or 20I/501Y.V1)-
This variant was first identified in late 2020 in the United Kingdom (Southwest England) and was temporally associated with an increase in regional infections.13,14 B.1.1.7 contains more than a dozen (including six key) mutations compared with other circulating strains, and subsequently has been identified in scores of other countries, including the United States. Several unpublished studies indicate this variant has increased transmissibility-perhaps by 50%-although the underlying mechanism is unknown. Because B.1.1.7 has increased transmissibility, adherence to prevention efforts is particularly important to reduce exposure and a potential case surge in 2021; expanded vaccination coverage could attenuate the impact. Moreover, B.1.1.7 may be associated with greater disease severity, although data are preliminary and unpublished. Several UK data analyses suggest the relative risk of mortality is higher for the UK variant compared with wild-type infection, although the absolute infection fatality rate remains low.15 Thus far, no evidence exists that the current vaccines are less effective against B.1.1.7.16
South African variant (VOC B.1.351 or 20H/501.V2)-
This variant was identified in late 2020 in the Republic of South Africa (RSA).17 RSA surveillance data indicate B.1.351 rapidly became the dominant local strain, suggesting its potential increased transmissibility. Also, B.1.351 has been identified in dozens of other countries, including the United States. Preliminary reports suggest B.1.351 decreases neutralizing antibodies after exposure, which could limit immunity and protection from reinfection.18 Plasma from recipients of the mRNA COVID-19 vaccines appears to maintain neutralizing activity against the RSA variant, but at lower antibody titers than with the wild-type virus.19 The clinical implications of these reductions in neutralizing activity are uncertain.
Brazil variant (VOC P.1 or 20J/501Y.V3)-
This variant was first identified in late 2020 in Japan in four travelers from Brazil, and subsequently was reported to account for 42% of specimens in Brazil’s Amazonas state in December 2020.20 P.1 subsequently has been identified in several other countries, including the United States. This mutation raises concerns about the potential for increased transmissibility and an impact on immunity.
California variants (VOCs B.1.427 and B.1.429)-
These variants appear to spread more easily, increasing transmissibility by 20%.21 There also appears to be a small reduction in the effectiveness of antibodies generated by a previous COVID-19 infection or COVID-19 vaccine.
G614 or D614G mutation-
All five of the aforementioned variants have the same key G614 or D614G mutation.22 A study that monitored amino acid changes in the spike protein of SARS-CoV-2 identified a glycine for aspartic acid substitution that became the dominant polymorphism globally over time.23 Laboratory evidence indicated variants with the G614 mutation had higher infectious virus levels in a human epithelial lung tissue culture system as well as higher upper respiratory tract infection in an animal model.24 While emergence of G614 as a common key mutation in dominant variants could be related to relative infectiousness, the clinical implications of these findings remain uncertain. The G614 mutation variants do not appear to be associated with a higher risk of hospitalization.23 The mutation also does not impact anti-spike antibody binding.25
Entry Receptor
SARS-CoV-2 interacts with the host cells’ angiotensin-converting enzyme 2 (ACE2) receptor, which is highly expressed in the heart, lungs, kidney, and gastrointestinal tract playing a key role in various cardiovascular and immune pathways. When the SARS-CoV-2 S protein binds to ACE2, the infection is triggered and develops clinical symptoms according to the entry receptor’s location in organs.10,26–29 High affinity of the virus to ACE2, 10 to 20 times higher than SARS-CoV, explains the efficient spread and transmission from person to person that has been reported to date.26,30 The higher level of circulating ACE2 in men than women, as well as in patients with diabetes or cardiovascular diseases, resulted in male patients and those with comorbidities having higher COVID-19 hospitalization and mortality rates.28,31
Transmission Mode
Like other respiratory infectious diseases, COVID-19 is mainly transmitted from infected (symptomatic and asymptomatic) individuals to close contacts while coughing, sneezing, touching, talking, singing, and laughing through airborne infectious particles and droplets,14,32–34environmental and cross-contamination,35 and potential fecal shedding.35,36 Evidence suggests that each primary infected individual on average causes two to three secondary infectious cases.7
The average number of secondary infections per primary case when a contagious person travels in the general population is known as the basic reproductive number, denoted as R0, and applies before any public health measures are enacted and before infections become widespread. The effective reproductive number, Re, is used for regular periodic monitoring (eg, 7-day average) during the course of an epidemic or pandemic accounting for contact behavioral changes, pharmaceutical and nonpharmaceutical interventions, and reduced susceptible population. When Re is <1, growth in cases slows; but when Re is >1, cases grow with 2 ≤ Re≤ 4 corresponding to exponential growth and rapid transmission, where cases double about every 5 days.
Particle Sizes and Transmission Routes
Two particle sizes and three transmission routes need to be considered when treating patients with respiratory infectious diseases to prevent transmission.37,38
Particle size-
(1) Respirable particles or droplet nuclei (≤10 μm) can be inhaled and reach the alveolar region. (2) Inspirable particles (10–100 μm) can be inhaled but cannot penetrate the alveolar region.
Transmission routes-
(1) Contact transmission is either directly from an infected person to another person or indirectly through an intermediate contaminated object. (2) Droplet spray is from person to person through respirable and inspirable particles. (3) Aerosol transmission is person-to-person transmission through airborne aerosolized particles, which can be inhaled into the oronasopharynx, trachea, and lung or can land in mucus membranes of the mouth, nose, and eyes. Transmission via feces and urine has been considered as potential SARS-CoV, MERS-CoV, and SARS-CoV-2 routes.
The presence of SARS-CoV-2 in aerosols has been shown to stay viable for 3 hours,39 and it could land in other individuals’ mucus membranes of the mouth, nose, and eye and develop related infections or land on surfaces and cause cross-contamination. Evidence shows the virus as less stable on copper and cardboard surfaces than on plastic and stainless-steel ones, where they were detectable up to 72 hours later.1,35,40
In hospital settings, pathogens could be transmitted directly as a sequela of various procedures, such as intubation (resulting in ventilator-associated pneumonia), cardiopulmonary resuscitation, bronchoscopy, autopsy, and surgery, with high-speed aerosol-generating devices exposing healthcare professionals and patients to infected particles. In dental settings, various procedures produce droplets, spatter, and aerosol-spreading infectious particles, increasing the risk of cross-contamination and exposing dental professionals and patients to virus.40
Transmission From Asymptomatic Individuals
Evidence shows COVID-19 transmits during pre-symptomatic and asymptomatic phases like some other diseases.41 Pre-symptomatic and asymptomatic persons have positive tests with mild or no symptoms and are capable of continuing social interactions and simultaneously transmitting disease to others, including their family members.1,36,41–43 Unfortunately, because pre-symptomatic and asymptomatic individuals cannot be identified without testing, the transmission rate from this group to others is uncertain.41 Studies have estimated their highly variable transmission rate as 0−70%, which depends on individuals’ behaviors.41
Clinical Symptoms
While the incubation period has been shown to range widely,44 it is currently estimated as 0 to 24 days before the onset of clinical symptoms,45 with a median of 2 to 7 days in various studies.31 During this period, asymptomatic individuals can transmit the virus to household and community members.45 Clinical COVID-19 symptoms have been modified since the pandemic began. In the first few months of the pandemic cases almost always started with coughing (dry) and/or fever1,5,11,28,42,46,47 with various symptoms such as sore throat,5,12,42 fatigue and body ache, nasal congestion,5,31,43 conjunctivitis,44,48 cholecystitis11 accompanied by diarrhea,5,11,28,31,46,49 runny nose,49 hepatic and gastrointestinal disorders, lymphopathy, and neurological diseases.7 Severe cases develop dyspnea (shortness of breath)1,47,49 and severe pneumonia.1,26,28,31,42,43,49,50 People who are elderly with underlying conditions are more prone to rapidly develop acute respiratory distress syndrome, septic shock, metabolic acidosis, and coagulation dysfunction, often resulting in death.31 The clinical course for the most seriously affected individuals in the United States and worldwide spans about 3 weeks on average; thus, population level data have shown surges in cases are followed by surges in hospitalizations about 1.5 weeks later and surges in mortality about another 1.5 weeks later (ie, median of about 3 weeks from case surges).51
Immunity Following Infection
Uncertainties remain about COVID-19 reinfection. Evidence from two sets of cases in Hong Kong and Nevada shows reinfection after recovering from first COVID-19 infection.52 However, more studies are needed to determine true reinfections versus positive polymerase chain reaction tests resulting from memory cells of a previous episode of an infection formed in the body.52
Prevention Strategies
In general, the key measure to reduce the spread of infectious particles is breaking the disease transmission chain. Considering the aforementioned potential COVID-19 transmission routes, various strategies should be followed to break the cycle at the community level and in healthcare settings. At the community level, personal and hand hygiene, use of personal protective equipment (PPE) (eg, facemasks, gloves, protective eyeglasses, etc), and physical (social) distancing play a critical role in breaking viral transmission routes and mitigating the disease onset, its consequences, and disease spread.53 Respiratory hygiene or cough etiquette, eg, covering one’s mouth using a medical mask, cloth mask, tissue, sleeve, or flexed elbow, when coughing or sneezing, followed by handwashing, could reduce the spread of infectious particles.14,54 A study showed that 40% of aerosols and 30% of respiratory droplets from COVID-19 positive cases were detectable when no mask was used, but when a surgical face mask was used 0% of SARS-CoV-2 was detectable in either the aerosol or the droplets.55
In healthcare settings, transmission routes are more diverse; in addition to person-to-person transmission, various medical and dental procedures generate airborne particles, increasing disease transmission risks as well as cross-contamination making the situation more demanding to control. Upon COVID-19’s emergence, health organizations such as the WHO, CDC, American Dental Association (ADA), Occupational Safety and Health Administration (OSHA), and public health authorities mandated that healthcare providers (HCPs), including dental care providers (DCPs), follow and optimize infection prevention and control (IPC) practices to break the disease transmission cycle. According to the CDC,in the context of COVID-19, IPC is vital in optimizing health systemsto keep patients and HCPs/DCPs healthy and safe.54
Infection Prevention and Control
Since March 2020, infection control guidelines have evolved as more information about SARS-CoV-2 transmission routes has been reported. The CDC has taken the lead to develop infection prevention and control (IPC) recommendations for both US and non-US healthcare settings. Various health organizations adopted an interim IPC guideline proposed to mitigate the COVID-19 spread and ensure safe practices for both patients and HCPs/DCPs.
Infection Prevention and Control in Dentistry and Hierarchy of Controls
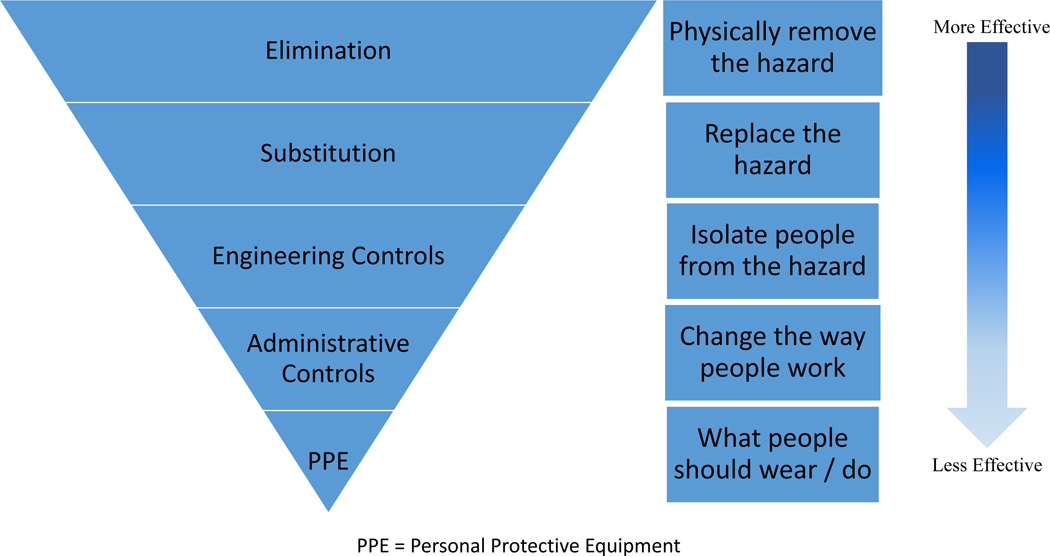
Because DCPs routinely can be exposed to airborne and bloodborne pathogens during routine dental care, dentistry is a healthcare profession that strictly follows infection control guidelines. However, no current HCPs had ever experienced anything like this pandemic.56 On May 19, 2020, the CDC released updated COVID-19 IPC guidance for dental settings that built upon infection control guidelines for DCPs introduced in 2003. Given COVID-19’s serious clinical sequelae, however, the CDC recommended using additional IPC to create safer practices. Aligning with CDC guidelines, the ADA suggested dentists postpone non-urgent dental procedures and only provide care for pain and infection using stringent IPC practices. The National Institute of Occupational Safety and Health (NIOSH) suggested a hierarchy of controls that emphasizes the importance of practicing IPC guidelines in mitigating COVID-19 spread (Figure 1).57 This hierarchy of controls is described as follows:
Figure 1:
Hierarchy of Controls. Adapted from NIOSH.
Personal protective equipment (PPE)-
Although this is the first step in the hierarchy, it is the least effective way to protect HCPs/DCPs. The use of N95 respirators instead of regular surgical masks is the new routine for DCPs in addition to standard PPE (gown, gloves, eye protection, faceshield, etc) that was used pre-COVID-19. Some DCPs prefer to also use hoods, coveralls, and shoe/hair covers to feel even safer in case they are providing care to pre-symptomatic/asymptomatic patients. According to NIOSH, PPE is used where hazards are not particularly well controlled.
Administrative controls-
Also called work practice controls, these aim to reduce HCP/DCP exposure to specific hazards by changing work processes when it is impractical to implement higher-level steps (ie, engineering controls, substitution, and elimination). Examples of administrative controls that can make the dental practice safer include safety training of DCPs, standardizing procedures, maintaining schedules for hazardous machines, housekeeping (eg, sustaining a clean and clutter-free space), redesigning the clinic reception area and furniture setting, and posting wall/floor signs to direct patients. It should be noted that due to the high risk of human error, administrative controls are not as effective as other controls; they are usually temporary, rather than long-term, solutions.
While both PPE and administrative controls are inexpensive resolutions initially, they can become relatively costly over the long term.
Engineering controls-
Third in the hierarchy, this solution requires a physical change to remove hazards (airborne and bloodborne pathogens) at the source before DCPs encounter them. Engineering controls are important to providing overall safe and healthy practices. Examples in dental care settings include the use of self-sheathing needles and safer medical devices, sharps disposal containers, proper ventilation systems (eg, high-efficiency particulate air [HEPA] filters), and high-volume evacuation (HVE). Although the initial installation of well-designed engineering controls is expensive compared to the implementation of administrative controls or PPE, over the longer term, operating costs become lower, and cost savings may result from the elimination of adverse effects and human errors (eg, occupational exposures).
Substitution-
Considered to be the second most effective method in the hierarchy of controls in protecting HCPs/DCPs, substitution replaces a hazardous process or material with a safe one. In dental settings, substitution techniques may include using minimally invasive protocols to reduce spatter, spray, and aerosol production; applying atraumatic restorative treatment protocols instead of using a high-speed handpiece to prepare and repair a decayed tooth; or using hand scalers instead of an ultrasonic scaler.
Elimination-
Finally, elimination is the most effective practice to protect HCPs/DCPs against infectious diseases and hazardous situations by physically removing the hazard from the workspace. For example, implementing a screening protocol to check both patients and employees before they arrive at the clinic is a simple yet effective way to reduce or eliminate transmission risk in healthcare settings.
Table 2 summarizes the main IPC guidelines to be aligned with the hierarchy of controls in dental care settings to enhance safer practice.
Table 2-.
ADA Infection Control and Prevention Guidelines for Dental Care Settings
| 1. Consider whether elective procedures, surgeries, and non-urgent outpatient visits should be postponed in certain circumstances. |
| 2. Implement telehealth triage protocols. |
| 3. Screen and triage everyone entering dental care settings for signs and symptoms of COVID-19. |
| 4. Implement universal source control measures (e.g., mandatory face masks). |
| 5. Encourage physical (social) distancing in the facility. |
| 6. Consider performing targeted SARS-CoV-2 testing of patients without signs or symptoms of COVID-19 |
| 7. Put administrative controls and work practices into effect. |
| 8. Incorporate universal use of personal protective equipment (PPE) and employ PPE supply optimization strategies |
| 9. Emphasize hand hygiene. |
| 10. Give consideration to how equipment is used. |
| 11. Optimize using engineering controls and indoor air quality. |
| 12. Maintain environmental infection control. |
Conclusion
This review outlined the SARS-CoV-2 virology, transmission routes, clinical symptoms, and epidemiology and briefly provided infection prevention and control strategies to be practiced in dental care settings. Giving consideration to the hierarchy of infection controls could assist dental care providers in ensuring delivery of safe dental care to their patients and reducing the disease transmission.
Case studies show dental practices can increase hygiene production at low cost with little effort. Discover these additional services that dynamically improve dental auxiliaries workflows and overall patient satisfaction.
Acknowledgments
Disclosure
Dr. Banava has been supported by an institutional NIH/NIDCR award T32DE007306 at the University of California San Francisco as part of her postdoctoral program. She and Dr. Gansky have been supported by University of California Office of the President (UCOP) Emergency COVID-19 Research Seed Funding through the California Breast Cancer Research Program (CBCRP) grant R00RG2901. The funders played no role in determining the methods or conduct of this study.
Dr. Gansky’s brother is an employee at 3M, which develops and markets PPE products; however, his brother does not fund research and had no role in the content of this article.
Contributor Information
Sepideh Banava, Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California San Francisco, San Francisco, California.
Stuart A. Gansky, Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California San Francisco, San Francisco, California; Affiliate Member, Philip R. Lee Institute for Health Policy Studies, UCSF.
Michael S. Reddy, Department of Orofacial Sciences, School of Dentistry, University of California San Francisco, San Francisco, California.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-19) pandemic. WHO website. Updated April27, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.Accessed April 27, 2021. [Google Scholar]
- 3.Johns Hopkins University of Medicine. Coronavirus Resource Center. Johns Hopkins Coronavirus Resource Center website. April 27, 2021. https://coronavirus.jhu.edu/.Accessed April 27, 2021. [Google Scholar]
- 4.Worldometer. Coronavirus. Worldometer website. April 27, 2021. https://www.worldometers.info/coronavirus/country/us/.Accessed April 27, 2021. [Google Scholar]
- 5.Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worldometer. Coronavirus. Worldometer website. March 20, 2021. https://www.worldometers.info/coronavirus/#countries.Accessed March 20, 2021. [Google Scholar]
- 7.Maiuolo J, Mollace R, Gliozzi M, et al. The contribution of endothelial dysfunction in systemic injury subsequent to SARS-CoV-2 infection. Int J Mol Sci. 2020;21(23):9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Risk for COVID-19 Infection, Hospitalization, and Death By Race/Ethnicity. CDC website. April 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html.Accessed April 27, 2021. [Google Scholar]
- 9.Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses - a statement of the Coronavirus Study Group. Nat Microbiol. 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control. Rapid increase of a SARS-CoV-2 variant with multiple spike protein mutations observed in the United Kingdom. ECDC: Stockholm; December 20, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf.Accessed April 27, 2021. [Google Scholar]
- 14.Centers for Disease Control and Prevention. About Variants of the Virus that Causes COVID-19. CDC website. April 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html.Accessed April 27, 2021. [Google Scholar]
- 15.Public Health England. Investigation of novel SARS-CoV-2 variant: Variant of Concern 202012/01. Technical briefing 5. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/957504/Variant_of_Concern_VOC_Accessed April 27, 2021.
- 16.Muik A, Wallisch A-K, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371(6534):1152–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. SARS-CoV-2 Variants. WHO website. December 31, 2020. https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/.Accessed April 27, 2021. [Google Scholar]
- 18.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27(4):622–625. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021;27(4):620–621. [DOI] [PubMed] [Google Scholar]
- 20.do Nascimento VA, Corado ALG, do Nascimento FO, et al. Genomic and phylogenetic characterisation of an imported case of SARS-CoV-2 in Amazonas State, Brazil. Mem Inst Oswaldo Cruz. 2020;115:e200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng X, Garcia-Knight MA, Khalid MM, et al. Transmission, infectivity, and antibody neutralization of an emerging SARS-CoV-2 variant in California carrying a L452R spike protein mutation [preprint]. medRxiv. 2021March9. doi: 10.1101/2021.03.07.21252647. [DOI] [Google Scholar]
- 22.Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. CDC website. April 26, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html.Accessed April 27, 2021. [Google Scholar]
- 23.Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plante JA, Liu Y, Liu J, Xia H, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klumpp-Thomas C, Kalish H, Hicks J, et al. D614G spike variant does not alter IgG, IgM, or IgA spike seroassay performance [preprint]. medRxiv. 2020. doi: 10.1101/2020.07.08.20147371. [DOI] [Google Scholar]
- 26.Walls AC, Park Y-J, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanff TC, Harhay MO, Brown TS, et al. Is there an association between COVID-19 mortality and the renin-angiotensin system? A call for epidemiologic investigations. Clin Infect Dis. 2020;71(15):870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge H, Wang X, Yuan X, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39(6):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharyya A, Seth A, Srivast N, et al. Coronavirus (COVID-19): a systematic review and meta-analysis to evaluate the significance of demographics and comorbidities [preprint]. Res Sq. 2021;rs.3.rs-144684. [Google Scholar]
- 32.Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441–442. [DOI] [PubMed] [Google Scholar]
- 33.Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2020;71(9):2311–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Anderson N, Pan Y, et al. The SARS-CoV-2 outbreak: diagnosis, infection prevention, and public perception. Clin Chem. 2020;hvaa080. doi: 10.1093/clinchem/hvaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel: Update 2010. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 38.O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13(15):3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ADA Center for Professional Success. Coronavirus Frequently Asked Questions. ADA website. https://success.ada.org/en/practice-management/patients/coronavirus-frequently-asked-questions.Accessed April 26, 2021. [Google Scholar]
- 41.Espinoza B, Marathe M, Swarup S, Thakur M. Adaptive human behavior in epidemics: the impact of risk misperception on the spread of epidemics [preprint]. Res Sq. 2021;rs.3.rs-220773. [Google Scholar]
- 42.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 2020;323(13): 1243–1244. [DOI] [PubMed] [Google Scholar]
- 44.Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology. 2006;212(suppl 1):119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323(13):1245–1246. [DOI] [PubMed] [Google Scholar]
- 48.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adalja AA, Toner E, Inglesby TV. Priorities for the US health community responding to COVID-19. JAMA. 2020;323(14):1343–1344. [DOI] [PubMed] [Google Scholar]
- 51.Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020;26(6):1339–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mustapha JO, Abdullahi IN, Ajagbe OOR, et al. Understanding the implications of SARS-CoV-2 reinfections on immune response milieu, laboratory tests and control measures against COVID-19. Heliyon. 2021;7(1):e05951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.van Eijk LE, Binkhorst M, Bourgonje AR, et al. COVID-19: immunopathology, pathophysiological mechanisms, and treatment options. J Pathol. 2021; 10.1002/path.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. COVID-19 Overview and Infection Prevention and Control Priorities in non-US Healthcare Settings. CDC website. February 26, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html.Accessed April 27, 2021. [Google Scholar]
- 55.Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks [published correction appears in Nat Med. 2020;26(6):981]. Nat Med. 2020;26(5):676–680.32371934 [Google Scholar]
- 56.Kathree BA, Khan SB, Ahmed R, et al. COVID-19 and its impact in the dental setting: a scoping review. PLoS One. 2020;15(12):e0244352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institute for Occupational Safety and Health. Hierarchy of Controls. Centers for Disease Control and Prevention/NIOSH website. January 13, 2015. https://www.cdc.gov/niosh/topics/hierarchy/default.html.Accessed April 27, 2021. [Google Scholar]