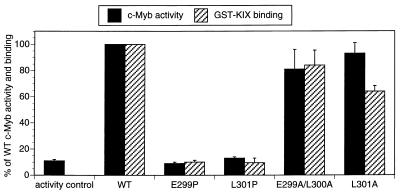
FIG. 4.
Helical structure of the c-Myb activating region is critical for complex formation with KIX and for target gene activation. The bar graph shows KIX binding activity of full-length wild-type (WT) and mutant c-Myb polypeptides (stippled bars). Effects of mutations with the helix-sparing amino acid alanine and the helix-breaking amino acid proline on KIX binding activity are shown. Binding of 35S-labeled c-Myb polypeptides to GST-KIX resin was quantitated and expressed as the percentage of WT c-Myb binding activity. Transient luciferase assays were performed on 293 cells transfected with WT and mutant c-Myb polypeptides (hatched bars) containing alanine or proline substitutions at amino acid positions listed under each bar. Luciferase activities derived from the CD13/APN-Luc reporter gene were normalized to β-galactosidase activity derived from cotransfected RSV-β-Gal control plasmid and expressed as percentages of WT c-Myb activity. Results are shown as means ± standard deviations (n = 3). Mutant and WT c-Myb polypeptides were comparably expressed in transfected 293 cells (data not shown).