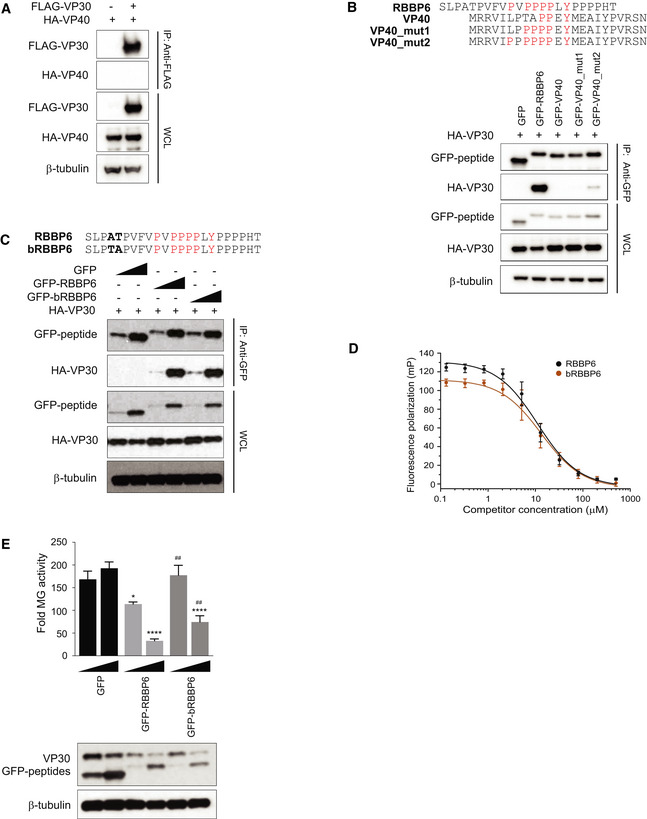
Co‐immunoprecipitation assay to assess HA–VP40 interaction with FLAG‐VP30. HEK293T cells were transfected with HA‐VP40 plasmid and either empty vector or FLAG‐VP30 plasmid. An anti‐FLAG immunoprecipitation (IP) was performed. IP and WCL were analyzed by Western blotting with anti‐HA and anti‐FLAG antibodies. An anti‐β‐tubulin Western blot provided a loading control.
Anti‐GFP immunoprecipitation of HA‐VP30 with GFP fusions to wild‐type or mutated VP40‐derived peptides (GFP‐VP40, GFP‐VP40_mut1, or GFP‐VP40_mut2). GFP without a fusion partner (GFP) and GFP fused to RBBP6 peptide (GFP‐RBBP6) served as controls. Immunoblots with anti‐HA, anti‐GFP, and anti‐β‐tubulin are shown.
GFP fused to peptides derived from human and bat (Rousettus aegyptiacus) RBBP6 were expressed along with HA‐VP30. Co‐immunoprecipitation was performed using anti‐GFP magnetic beads, and representative immunoblots for IP and WCL are shown.
Equilibrium dissociation curves of FITC‐RBBP6 peptide to eVP30130‐272 as it is outcompeted by increasing concentrations (0.13–500 μM) of human and bat RBBP6 peptides. Fluorescence polarization was determined with constant concentrations of FITC‐RBBP6 and eVP30130‐272, at 0.50 μM and 3.8 μM, respectively. Experiments were performed in two independent replicates. Error bars represent standard deviation.
MG activity upon titration of GFP fused to human and bat‐derived RBBP6 peptides (12.5 ng and 125 ng). GFP alone was used as a control. Reporter activity was read at 48 h post‐transfection and fold MG activity was calculated relative to a no VP30 control. # denotes statistical significance compared with RBBP6 peptide for each dose. The data represent the mean ± SD from one representative experiment in which each transfection condition was performed in triplicate (n = 3). Each experiment was reproduced in at least two additional, independent experiments (see Appendix Fig S1). Statistical significance was calculated relative to GFP control for each concentration tested using ANOVA with Tukey’s multiple comparisons test. ****P < 0.00005; ##
P < 0.005, *P < 0.05