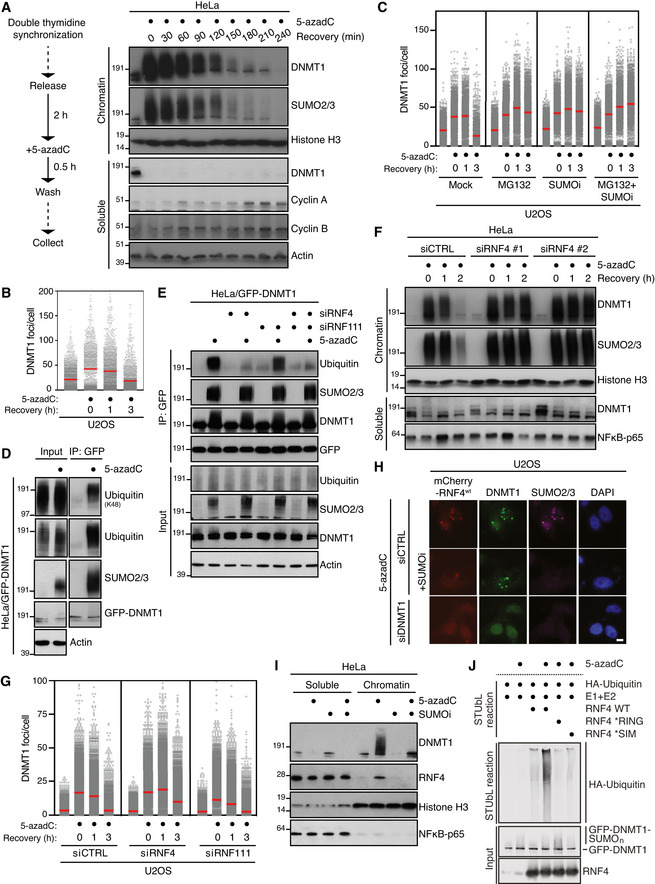
HeLa cells released from double thymidine synchronization in early S phase were treated with 5‐azadC for 30 min, washed and collected at the indicated times. Soluble and chromatin‐enriched fractions were immunoblotted with indicated antibodies.
U2OS cells treated as in (A) were pre‐extracted and immunostained with DNMT1 antibody. DNMT1 foci formation was analysed by quantitative image‐based cytometry (QIBC) (red bars, mean; > 1,500 cells analysed per condition). Data are representative of three independent experiments. See also Fig
EV1A.
As in (B), except that cells were pre‐treated with the proteasome inhibitor MG132 and/or SUMOi for 30 and 15 min, respectively, before exposure to 5‐azadC (red bars, mean; > 1,900 cells analysed per condition). Data are representative of three independent experiments.
HeLa cells stably expressing GFP‐DNMT1 were treated or not with 5‐azadC for 30 min, collected and subjected to GFP immunoprecipitation under denaturing conditions, and immunoblotted with indicated antibodies.
HeLa/GFP‐DNMT1 cells transfected with indicated siRNAs were processed as in (D).
Immunoblot analysis of soluble and chromatin‐enriched fractions of HeLa cells transfected with indicated siRNAs, left untreated or exposed to 5‐azadC for 30 min and collected at the indicated times.
U2OS cells transfected with indicated siRNAs were treated with 5‐azadC for 30 min and processed for QIBC analysis of DNMT1 foci counts as in (B) (red bars, mean; > 5,600 cells analysed per condition). Data are representative of three independent experiments.
Representative images of U2OS cells transfected with indicated siRNAs followed by mCherry‐RNF4 expression plasmid. Cells were treated with 5‐azadC in the presence or absence of SUMOi, fixed 2 h later, pre‐extracted and co‐immunostained with DNMT1 and SUMO2/3 antibodies. Scale bar, 10 µm.
Immunoblot analysis of soluble and chromatin‐enriched fractions of HeLa cells treated with 5‐azadC and/or SUMOi as indicated.
GFP‐tagged DNMT1 from extracts of HeLa/GFP‐DNMT1 cells treated or not with 5‐azadC was immobilized on GFP‐Trap agarose, subjected to stringent washing to remove proteins non‐covalently bound to GFP‐DNMT1 and incubated with recombinant HA‐ubiquitin, E1 and E2 (UbcH5a) enzymes and RNF4 proteins (STUbL reaction) at 37°C for 1 h. Samples were then subjected to immunoblotting to assay for RNF4‐dependent STUbL activity towards GFP‐DNMT1.