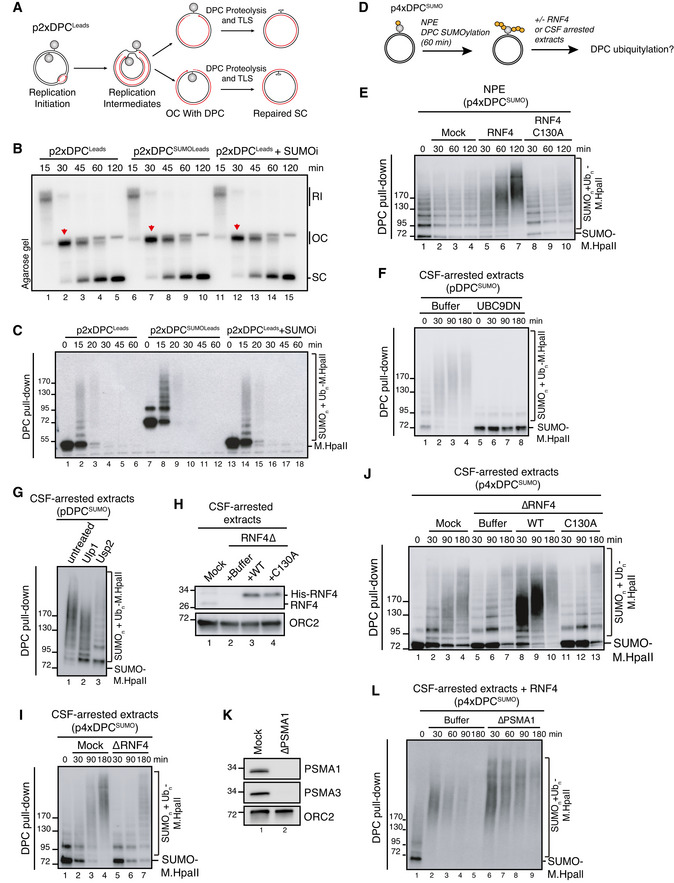
Scheme illustrating replication in egg extracts of p2xDPC
Leads, a plasmid containing two M.HpaII crosslinked on opposite strands. Under these conditions, the vast majority of replication forks encounter the DPC on their leading strand template (Larsen
et al,
2019).
p2xDPCLeads or p2xDPCSUMOLeads were replicated in egg extracts in the presence [α‐32P]dATP. Where indicated, 50 µM of SUMOi was added to the extracts. Reaction samples were analysed by native agarose gel electrophoresis. RI, replication intermediates; OC, open circular; SC, supercoiled. Red arrowheads indicate OC molecules that have not yet undergone repair.
Samples in (B) were recovered by DPC pull‐down and immunoblotted for crosslinked M.HpaII.
Scheme illustrating sequential SUMOylation and ubiquitylation of DPC‐containing plasmids in non‐replicating egg extracts. First, p4xDPCSUMO is incubated in NPE for 60‐90 min to achieve poly‐SUMOylation of the DPCs. NPE is then supplemented with recombinant RNF4 to a final concentration of 7 ng/µl (E) or an equal volume of CSF‐arrested whole egg extract (F, G) to trigger DPC ubiquitylation.
p4xDPCSUMO was incubated in NPE for 60 min and supplemented with buffer or recombinant RNF4 (WT or a catalytically inactive C130A mutant). At the indicated time points following RNF4 addition, the DPC plasmid was recovered by DPC pull‐down and immunoblotted against M.HpaII.
pDPCSUMO was subjected to sequential extract addition as depicted in (D). Recombinant UBC9DN was added to NPE where indicated to block de novo SUMOylation. At the indicated time points following addition of CSF‐arrested egg extract, the plasmid was recovered via DPC pull‐down and immunoblotted against M.HpaII.
pDPCSUMO was subjected to sequential extract addition as depicted in (D). Ninety min after addition of CSF‐arrested extract, samples were recovered via DPC pull‐down and treated with the SUMO protease Ulp1 or the ubiquitin protease Usp2 as indicated. Samples were then immunoblotted against M.HpaII.
CSF‐arrested extracts were either mock‐ or RNF4‐depleted and recombinant His‐RNF4 was supplemented to RNF4‐depleted extracts to a final concentration of 7 ng/µl where indicated. Protein samples were immunoblotted with the indicated antibodies.
p4xDPCSUMO was polySUMOylated in NPE, recovered via DPC pull‐down and incubated in fresh CSF‐arrested extract that was either mock‐ or RNF4‐depleted. At indicated time points following CSF extract addition, the plasmid was recovered and immunoblotted against M.HpaII.
As in (I), but using RNF4‐depleted CSF‐arrested extracts reconstituted with recombinant RNF4 WT or C130A from (H).
CSF‐arrested extracts were either mock‐ or PSMA1‐depleted. Protein samples were immunoblotted with the indicated antibodies.
p4xDPCSUMO was subjected to sequential addition of NPE and CSF‐arrested extract as in (I). CSF‐arrested extracts from (K) were supplemented with recombinant RNF4, and the DPC plasmid was recovered by DPC pull‐down at the indicated time points and immunoblotted against M.HpaII.