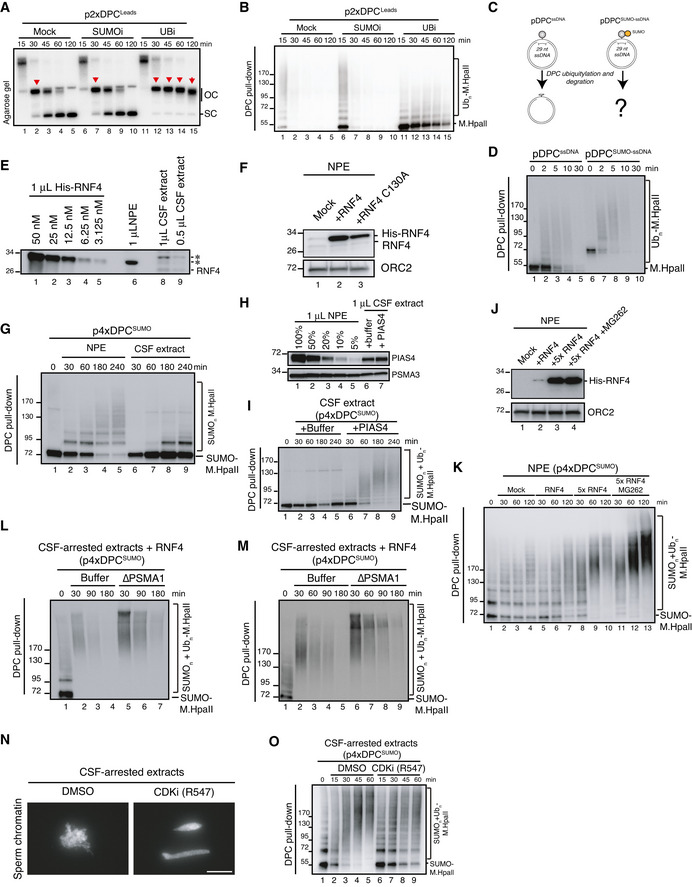
-
A
p2xDPC
Leads was replicated in egg extracts in the presence of radiolabelled nucleotides in the presence or absence of the SUMO E1 or ubiquitin E1 inhibitors. Samples were analysed by native agarose gel electrophoresis as in Fig
3B. Red arrowheads indicate open circular molecules (OC) that have not yet undergone repair.
-
B
Samples from (A) were recovered by DPC pull‐down and immunoblotted against M.HpaII. Note that in the presence of the ubiquitin E1 inhibitor DPC ubiquitylation and degradation by both SPRTN and the proteasome are severely inhibited (Duxin
et al,
2014; Larsen
et al,
2019). Residual ubiquitylation likely reflects ubiquitin E1 enzymes in the extract activated prior to addition of the inhibitor.
-
C
Scheme illustrating DPC repair in egg extracts when M.HpaII or SUMO‐M.HpaII is crosslinked to ssDNA (pDPC
ssDNA or pDPC
SUMOssDNA). In this setting, DPC ubiquitylation is mainly driven by the E3 ubiquitin ligase RFWD3 and does not require DNA replication (Gallina
et al,
2021).
-
D
pDPCssDNA or pDPCSUMO‐ssDNA were incubated in non‐replicating egg extracts. DPCs were recovered by pull‐down and immunoblotted against M.HpaII. Note the similar kinetics of degradation for SUMOylated and non‐SUMOylated DPCs.
-
E
Indicated volumes of whole egg CSF‐arrested extract and NPE were immunoblotted with RNF4 antibody next to a dilution series of recombinant His‐RNF4. A band migrating around 28 kDa and immunodepleted with the RNF4 antibody (Fig
3H) is indicated as RNF4. * denotes non‐specific bands that are not immunodepleted by the RNF4 antibody.
-
F
Samples from Fig
3E were immunoblotted with the indicated antibodies.
-
G
p4xDPCSUMO was incubated in NPE or CSF‐arrested extracts. At the indicated times, the DPCs were recovered and immunoblotted against M.HpaII.
-
H
Indicated volumes of whole egg CSF‐arrested extract and NPE were immunoblotted with PIAS4 and PSMA3 antibodies. Where indicated, CSF‐arrested extract was supplemented with 10 ng/µl of recombinant PIAS4.
-
I
p4xDPCSUMO was incubated with CSF‐arrested extracts from (H). DPCs were recovered at the indicated time points and immunoblotted against M.HpaII.
-
J
p4xDPCSUMO was incubated in NPE for 60 min and supplemented with buffer or either 7 ng/µl or 35 ng/µl (5×) of recombinant RNF4 (WT) in the presence of MG262 where indicated. Protein samples were immunoblotted with the indicated antibodies.
-
K
Samples from (J) were recovered via DPC pull‐down at the indicated time following RNF4 addition and immunoblotted against M.HpaII.
-
L, M
Independent replicates of Fig
3L.
-
N
Sperm chromatin was incubated for 1 h in CSF‐arrested egg extract in the presence or absence of 10 µM of the pan‐CDK inhibitor R547. Note that chromosome condensation is inhibited in the presence of R547, consistent with CDK inhibition. Scale bar, 30 µm.
-
O
Sequential addition of Xenopus egg extracts to DPC‐containing plasmids. After the first addition of NPE, the SUMOylated DPCs were recovered and incubated in CSF‐arrested egg extract in the presence or absence of R547. The DPCs were again recovered and immunoblotted against M.HpaII.