Figure 1. Classical EMT functions and cancer.
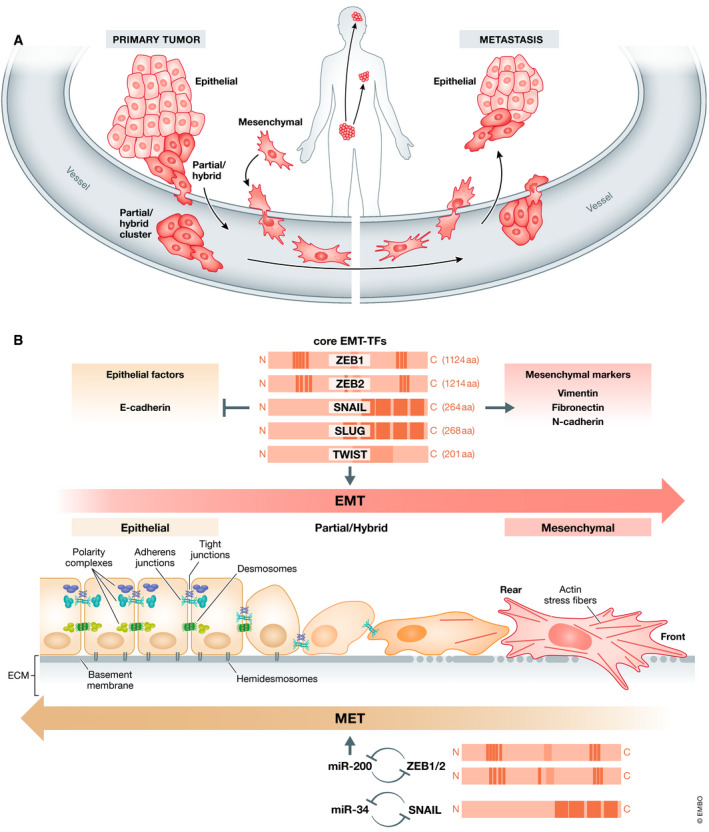
(A) EMT frequently occurs at the invasive front of epithelial tumors, destroys the well‐defined epithelial structures, and allows the cancer cells to migrate, invade the tissue, and intravasate in blood or lymphatic vessels. Tumor cells on their way through the body can travel as mesenchymal single cells, as cell clusters exhibiting partial EMT or as more epithelial cell clusters headed by a mesenchymal leader cell. At the secondary site, the cells extravasate and colonize the distant organ, where MET allows the outgrowth to macrometastases. (B) EMT is induced mainly by a set of transcription factors (EMT‐TFs) like ZEB1, ZEB2, SNAIL, SLUG and TWIST that differ in protein structure, size, and individual functions. All of them are repressors of epithelial factors like E‐cadherin and activate mesenchymal markers like Vimentin, Fibronectin or N‐cadherin. Epithelial cells displaying apical–basal polarity are held together by tight junctions, adherens junctions, and desmosomes and are anchored to the underlying basement membrane by hemidesmosomes. They express three different polarity complexes that together with the junctional molecules maintain epithelial cell polarity. In the classical EMT, expression of EMT‐TFs leads to inhibition of major components of these epithelial structures and concomitantly activates the expression of genes associated with the mesenchymal state. Cells gain front–rear polarity, display actin stress fibers, become motile and acquire invasive capacities. Notably, tumor cells very rarely switch to a completely mesenchymal phenotype, but fluently convert between various intermediate states displaying certain mesenchymal features but keeping partial sets of epithelial characteristics. Further, EMT is a reversible process. Mesenchymal cells can revert to the epithelial state undergoing MET. An important role in the execution of MET is played by microRNAs of the miR‐200 and mir‐34 families that are regulated in double‐negative feedback loops with the EMT‐TFs ZEB1/2 and SNAIL, respectively, that serve to reinforce either the epithelial or the mesenchymal state.
