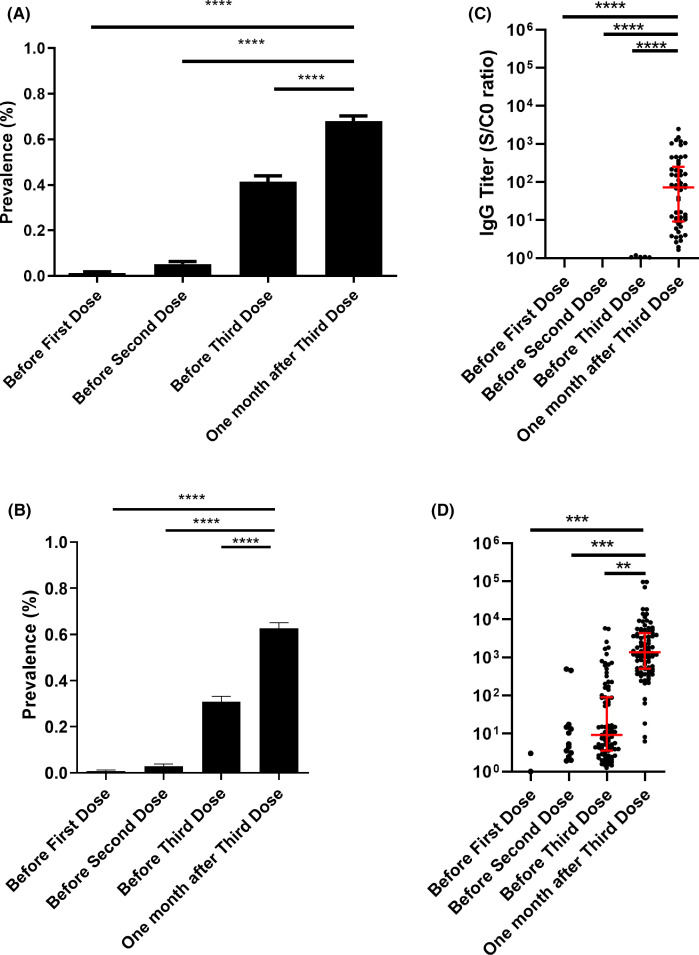
FIGURE 1.
(A) Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies before and after vaccination in patients tested with different assays. Results are expressed as means ± SEM. ****p < .0001. (B) Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies before and after vaccination in patients tested with the Wantai enzyme-linked immunosorbent assay test. Results are expressed as means ± SEM. ****p < .0001. (C) Anti-SARS-CoV-2 antibodies titers before and after vaccination in patients who were tested with the Wantai test and who had no detectable antibodies titers before the third dose. A positive test using the Wantai assay was defined by a signal-to-cut-off ratio (S/CO) >1.1. ****p < .0001. (D) Anti-SARS-CoV-2 antibody titers before and after vaccination in patients who were tested with the Wantai test and who had detectable antibodies titers before the third dose. Results are expressed as means ± SD. A positive test using the Wantai assay was defined by a S/CO >1.1. ***p = .004. **p = .017 [Color figure can be viewed at wileyonlinelibrary.com]