Abstract
Background
Perinuclear anti‐neutrophil cytoplasmic antibodies (P‐ANCA) are associated with a multisystem vasculitis affecting small blood vessels in the body. A handful of adult patients who developed vasculitis post‐COVID‐19 have been reported. Although SARS‐CoV‐2 has been shown to drive an exaggerated immune response in the pediatric population, such as in Multisystem Inflammatory Syndrome in Children (MIS‐C), only one case of vasculitis following COVID‐19 has been reported previously in children.
Case presentation
Seventeen‐year‐old male with a past medical history of COVID‐19 pneumonia two months prior presented with acute kidney injury and diffuse alveolar hemorrhage. Rheumatologic workup revealed P‐ANCA and Myeloperoxidase (MPO) positivity. Kidney biopsy showed necrotizing glomerulonephritis with limited immune complex deposition. Subsequently, he was treated with steroids and plasmapheresis, and ultimately started on cyclophosphamide.
Conclusions
To our knowledge, this report presents the second reported pediatric case of P‐ANCA/MPO vasculitis following COVID‐19.
Keywords: COVID‐19, diffuse alveolar hemorrhage, P‐ANCA vasculitis
Highlights
Autoimmune development postinfectious process.
Autoimmune disease and its association to SARS‐CoV‐2 infection.
Pediatric P‐ANCA vasculitis post COVID‐19
Abbreviations
- AKI
acute kidney injury
- BAL
bronchoalveolar lavage
- BUN/Cr units in mg/dl
blood urea nitrogen to creatinine ratio
- COVID‐19
coronavirus disease 2019
- FFB
flexible fiberoptic bronchoscopy
- FiO2
fraction of inspired oxygen
- Hb/Hct units in gm/dl and percent, respectively
hemoglobin/hematocrit
- HFNC
high flow nasal cannula
- IgG
immunoglobulin G
- LPM
liters per minute
- MPO
myeloperoxidase
- P‐ANCA
perinuclear anti‐neutrophil cytoplasmic antibodies
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. CASE DESCRIPTION
A seventeen‐year‐old male with a past medical history of obesity and asthma was hospitalized due to COVID‐19 pneumonia and respiratory insufficiency, requiring high flow nasal cannula (HFNC) up to 30 LPM, FiO2 50%. Chest X‐ray showed moderate bilateral infiltrates. His initial admission Hb/Hct were 9.1/27.5 with a nadir of 7.7/23.8, and BUN/Cr was 9/0.78 during this hospitalization. He was treated with Remdesivir, Dexamethasone, Azithromycin, Beclomethasone inhaled 80 mcg twice daily, and iron supplementation. Before discharge his chest X‐ray partly normalized and he was discharged home on room air. One month later, he was readmitted with elevated blood pressure, hematuria, and proteinuria, and diagnosed with acute kidney injury (AKI) with a BUN/Cr of 16/1.30. The patient's lowest Hb/Hct during this admission was 7.4/23.0. The AKI was thought to be due to dehydration, which improved after a few days of fluid management with subsequent normalization of his blood pressure. He was discharged on room air, with Hb/Hct of 7.9/24.8 and a BUN/Cr of 15/0.99.
One month after the second hospital admission, he presented to the emergency room with worsening cough, fatigue, exertional dyspnea, and amber‐colored urine. SARS‐CoV‐2 PCR was negative. He developed acute respiratory insufficiency requiring respiratory support with HFNC (40 LPM, FiO2 60%), AKI (BUN/Cr 30/1.52), and was revealed to have significant anemia (Hb/Hct 5.5/16.8). Computed tomography angiography of the chest showed no evidence of pulmonary embolus but did show extensive heterogeneous infiltrates in both lungs with an unusual fluffy central distribution concerning for diffuse alveolar hemorrhage (DAH) (Figure 1). Flexible fiberoptic bronchoscopy with bronchoalveolar lavage (FFB/BAL) demonstrated DAH as evidenced by the BAL return color and RBC presence, along with positive hemosiderin‐laden macrophages. Respiratory culture and PCR respiratory panel from BAL were negative for an infectious etiology including negative SARS‐CoV‐2 PCR. He then disclosed that he had a yearlong knee & lower back pain as well as generalized body aches, which were intensified after initial COVID‐19 infection. Rheumatologic evaluation revealed antinuclear antibody, perinuclear anti‐neutrophil cytoplasmic antibodies (P‐ANCA), and myeloperoxidase (MPO) antibody positivity, culminating in a diagnosis of ANCA positive vasculitis. Renal biopsy showed necrotizing glomerulonephritis with limited immune complex deposition.
Figure 1.
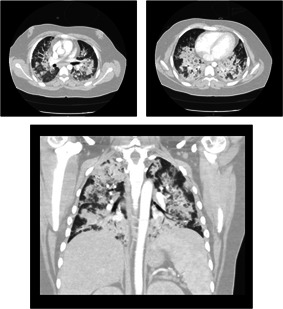
CT angiography noting extensive heterogeneous infiltrates in both lungs with an unusual fluffy central distribution concerning for diffuse alveolar hemorrhage
He was initially treated with methylprednisolone (1 g/day) for 72 h and was simultaneously started on plasmapheresis which he received for 5 consecutive days. Following plasmapheresis, he was started on cyclophosphamide infusions 10 mg/kg IV every 2 weeks and methylprednisolone was slowly weaned to 0.85 mg/kg/day over 7 days, and then was transitioned to oral steroids. DAH and AKI resolved, and he was discharged on room air without need for outpatient dialysis.
2. DISCUSSION
The precise etiology of autoimmune disorders is yet unclear, though there is growing evidence that there is a role for both genetic predisposition and environmental factors. 1 It is proposed that infections with bacterial or viral organisms trigger an exaggerated host immune response via molecular mimicry and activation of pre‐primed autoreactive T cells and proinflammatory mediators. 1 This in turn may lead to tissue damage and multisystem organ dysfunction. 1
SARS‐CoV‐2 has been shown to share similar pathogenic mechanisms driving an exaggerated immune response. 2 There is a growing evidence to suggest an association between prior SARS‐CoV‐2 infection and subsequent development of autoimmune disorders—primarily in the adult population—such as Systemic Lupus Erythematosus, Guillain–Barré syndrome, Kawasaki disease, and Rheumatoid Arthritis. 2 In the pediatric population the most reported autoimmune phenomenon associated with COVID‐19 is Multisystem Inflammatory Syndrome in Children (MIS‐C). 3
Acute P‐ANCA vasculitis following COVID‐19 is a rare but documented clinical presentation in the adult population. 4 Only one other pediatric case linking past SARS‐CoV‐2 infection with P‐ANCA and MPO positive small vessel vasculitis was reported previously. 5 This case also demonstrated acute renal injury/failure and DAH. These clinical manifestations are unlikely a to be a direct result of acute COVID‐19, as both patients had multiple negative COVID‐19 PCR tests while their COVID‐19 IgG was positive. Although our patient was reporting minor fatigue and body aches before COVID‐19 raises the possibility of pre‐existing vasculitis, he developed major systemic involvement including AKI and DAH only after his acute SARS‐CoV‐2 infection resolved. Therefore, SARS‐CoV‐2 infection is suspected to be the trigger—or at the very least a significant exacerbating factor—of the vasculitis in this case.
3. CONCLUSIONS
To our knowledge, this report presents the second reported pediatric case of P‐ANCA/MPO vasculitis following COVID‐19. There is a need for closer and more comprehensive monitoring of patients post COVID‐19, particularly of those demonstrating multisystem organ involvement. More research is required to better elucidate the link between SARS‐CoV‐2 and its ability to trigger and/or exacerbate autoimmune disorders, as well as their most appropriate medical management.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, collected clinical history and data, drafted the initial manuscript, reviewed, and revised the manuscript: Yaron Fireizen. Supervision, reviewed, and revised the manuscript: Bugsu Ovunc. Collected clinical history and data, reviewed and revised the manuscript: Nastasia Nianiaris. Reviewed and revised the manuscript: Cyrus Shahriary, Inderpal Randhawa, and Maria E. Imperial. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
INFORMED CONSENT
Informed consent was obtained from parent and patient. The privacy rights of human subjects were observed.
Fireizen Y, Shahriary C, Imperial ME, Randhawa I, Nianiaris N, Ovunc B. Pediatric P‐ANCA vasculitis following COVID‐19. Pediatric Pulmonology. 2021;56:3422‐3424. 10.1002/ppul.25612
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. 10.3390/v11080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID‐19. J Autoimmun. 2020;114:102506. 10.1016/j.jaut.2020.102506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abrams JY, Godfred‐Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review [published online ahead of print, 2020 Aug 5]. J Pediatr. 2020;226(45–54):e1. 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hussein A, Al Khalil K, Bawazir YM. Anti‐neutrophilic cytoplasmic antibody (ANCA) vasculitis presented as pulmonary hemorrhage in a positive COVID‐19 patient: a case report. Cureus. 2020;12(8):e9643. 10.7759/cureus.9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powell WT, Campbell JA, Ross F, Peña Jiménez P, Rudzinski ER, Dickerson JA. Acute ANCA vasculitis and asymptomatic COVID‐19. Pediatrics. 2021;147:e2020033092. 10.1542/peds.2020-033092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


