ABSTRACT
Host immune responses to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), especially in children, are still under investigation. Children with coronavirus disease 2019 (COVID‐19) constitute a significant study group of immune responses as they rarely present with severe clinical manifestations, require hospitalization, or develop complications such as multisystem inflammatory syndrome in children (MIS‐C) associated with SARS‐CoV‐2 infection. The deciphering of children’s immune responses during COVID‐19 infection will provide information about the protective mechanisms, while new potential targets for future therapies are likely to be revealed. Despite the limited immunological studies in children with COVID‐19, this review compares data between adults and children in terms of innate and adaptive immunity to SARS‐CoV‐2, discusses the possible reasons why children are mostly asymptomatic, and highlights unanswered or unclear immunological issues. Current evidence suggests that the activity of innate immunity seems to be crucial to the early phases of SARS‐CoV‐2 infection and adaptive memory immunity is vital to prevent reinfection.
Keywords: SARS‐CoV‐2, COVID‐19, Immunity, Multisystem inflammatory syndrome, Children
Short abstract
Despite the limited immunological studies from children with COVID‐19, this review compares data between adults and children in terms of innate and adaptive immunity to SARS‐CoV‐2, discusses the possible reasons why children are mostly asymptomatic, and highlights unanswered or unclear immunological issues.
1. Introduction
Coronaviruses (CoVs) are enveloped positive‐sense single‐stranded RNA viruses of Coronaviridae family1 which infect both animals and humans.2, 3 CoVs are classified into four genera: alpha, beta, gamma, and delta. The human coronaviruses (HCoVs) belong to two of these genera: alpha CoVs (HCoV‐229E and HCoV‐NL63) and beta CoVs [HCoV‐HKU1, HCoV‐OC43, Middle East respiratory syndrome coronavirus (MERS‐CoV), the severe acute respiratory syndrome coronavirus (SARS‐CoV)].4 Outbreaks had been exacerbated by SARS‐CoV and MERS‐CoV in 2003 and 2012, respectively, which were characterized by low transmission but high mortality rates.5
In late 2019, a novel coronavirus was identified as the primary cause of a significant number of pneumonia cases in Wuhan, China. In February 2020, the new CoV strain was named SARS‐CoV‐2 and the disease was designated coronavirus disease 2019 (COVID‐19) by World Health Organization (WHO).6
Not only adults, but also children of all ages are typically infected by SARS‐CoV‐2. Most reported cases of infected children were attributed to contact with an infected family member.7, 8 Viral transmission rates have also been reported in activities outside the household, such as schools and kindergarten.9, 10 However, the presence of children and teachers in the educational settings was not accompanied with an increased risk of viral transmission, compared to the broader community.10 The incidence rate of pediatric COVID‐19 cases ranges between 1%–5% of all COVID‐19 cases worldwide. However, this rate is likely to be underestimated, given the high proportion of underdiagnosed mild symptomatic and asymptomatic cases.11
Although most cases of pediatric COVID‐19 cases present as asymptomatic or mild,12 infants and children with underlying medical conditions often require hospitalization to prevent life‐threatening complications.13, 14 Severe post‐infection clinical manifestations in children include the multisystem inflammatory syndrome in children (MIS‐C) that resembles to Kawasaki disease (KD) or toxic shock syndrome (TSS) and develops weeks or months after the onset of COVID‐19 symptoms.15
To date, limited data regarding SARS‐CoV‐2 immune responses in children have been published, mainly due to their milder phenotype or the asymptomatic presentation of undiagnosed cases. The aim of this review is to present current evidence regarding innate, humoral, and cellular immune responses to SARS‐CoV‐2 infection in children, including the novel MIS‐C associated with COVID‐19 and to compare with immunological data in adults.
2. Mucosal immune response
As SARS‐CoV‐2 initially infects the upper respiratory tract, the mucosal immune response of the nasopharynx, including tonsils and adenoids, is activated.16 IgA antibodies play a safeguarding role in mucosal immunity of the upper and lower respiratory tract by eliminating viral replication and reducing the risk of reinfection.17, 18 There are three heterogenous molecular forms of IgA immunoglobulin: secretory, monomeric, and polymeric.16 Secretory IgA is dimeric, whereas circulating IgA is monomeric.16 Secretory IgA is dimeric, whereas circulating IgA is monomeric.16
Secretory IgA significance in lymphoid tissues cannot be ignored. In a recent study of 173 participants, including patients with COVID‐19 and a healthy group, approximately 15%–20% of seronegative patients with mild disease had detectable IgA antibodies with neutralizing activity in various mucosal sites, including tears, nasal fluid and saliva.19 A statistically significant negative correlation between IgA titers and age was detected.19
Large seroepidemiological studies have shown that IgA levels increase with age, with the highest levels being encountered in adolescence.20 It has already been established that the elevation of circulating IgA antibody levels in hospitalized adult COVID‐19 patients have been associated with worse prognosis and increased fatality rates.19, 21 However, Gruber et al22 highlighted the crucial role of mucosal immunity in SARS‐CoV‐2 by investigating its role in 9 MIS‐C patients. Interestingly, in MIS‐C patients, IgA antibody titers remained elevated in the convalescent phase of the disease with comparable levels to the acute phase.22 There was also a notable accordance between gastrointestinal clinical manifestations, mucosal immune dysregulation via IL‐17A stimulation, and mucosal chemotaxis via CCL20 and CCL28 activation.22
Children and adolescents are characterized by increased bronchus‐associated lymphoid tissue (BALT), which is often activated by infections, but it is rarely encountered in adults.23 Since children with COVID‐19 usually have mild clinical manifestations, without excluding severe complications from the respiratory tract, the role of BALT in SARS‐CoV‐2 infection and disease progress remains elusive and requires further investigation.16
3. Innate immunity
The first line of defense against pathogens is the innate immunity. The cells of the innate immune system are able to identify specific molecular patterns (pathogen‐associated molecular patterns, PAMPs) of microorganisms through various proteins named as pattern recognition receptors (PRRs) [Figure 1, (1)].24 These PAMPs, such as viral RNA and proteins are recognized by the transmembrane PRRs, toll‐like receptors (TLRs), which are expressed in the innate immune system cells, such as monocytes, macrophages, epithelial cells, neutrophils and dendritic cells [Figure 1, (1)].25, 26, 27
FIGURE 1.
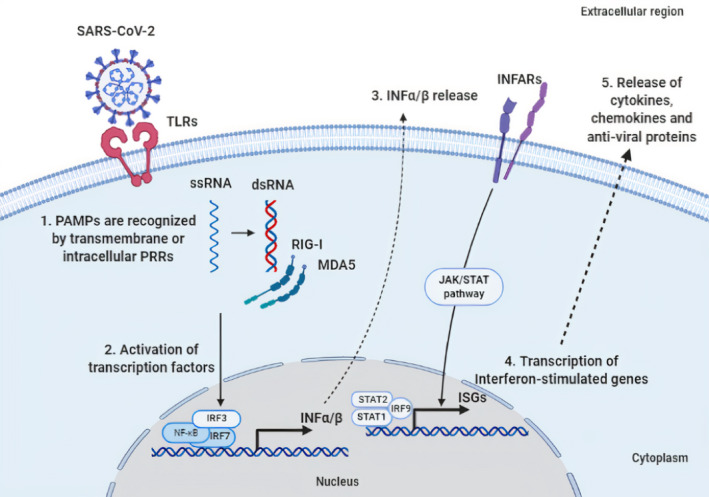
Basic molecular mechanisms of innate immune cells in response to SARS‐CoV‐2 infection. (1) Cells of the innate immune recognize pathogen‐associated molecular patterns (PAMPs), like viral proteins and double‐stranded RNA (dsRNA), through transmembrane or intracellular pattern recognition receptors (PRRs) such as toll‐Like receptors (TLRs) or RIG‐I‐like receptors (RLRs) consisting of the retinoic acid‐inducible gene‐I (RIG‐I) and the melanoma differentiation‐associated protein 5 (MDA5). (2) This recognition leads to the activation of the transcription factors, nuclear factor kappa B (NF‐κB), interferon regulatory factor 3 (IRF3), and IRF7, resulting in the expression of interferons (IFNs) α/β. (3) The binding of INF‐α/β to interferon‐alpha/beta receptor (INFAR) activates the JAK/STAT signaling pathway, which leads to (4) the expression of interferon‐stimulated genes (ISGs), by the signal transducer and activator of transcription 1 (STAT1), STAT2 and IRF9, and (5) finally to release of different cytokines, chemokines and anti‐viral proteins. The figure was designed by the biorender in silico tool (https://app.biorender.com/).
There are many TLRs that play an important role in COVID‐19 pathogenesis, including TLR2, TLR3, TLR4, TLR6, TLR7, TLR8, and TLR9, mainly activated by the stimulation of the proinflammatory cytokines IL‐1, IL‐6, and TNF‐α.28 Recent in silico studies showed that SARS‐CoV‐2 spike (S) protein, after being bound to angiotensin‐converting enzyme 2 (ACE2) receptor, activates a signaling inflammatory pathway through stimulating TLRs, especially TLR1, TLR4, and TLR6.29
PRR activation stimulates the secretion of cytokines, such as the following interleukins IL‐1, IL‐6, and IL‐18 [Figure 1, (2)]. Type I/III interferons (IFNs) induced by PRRs are considered to play a crucial role in early‐onset antiviral defense in SARS‐CoV‐2 and other viral infections [Figure 1, (3)‐(4)‐(5)]. Interestingly, certain type I IFN subtypes, IFN‐β1a and IFN‐β1b were proved to interfere effectively in SARS‐CoV in vivo inhibition and SARS‐CoV‐2 in vitro confrontation.30, 31
However, SARS‐CoV‐2 and other coronaviruses have invented several evasion mechanisms by inhibiting PRR stimulation and IFN signaling.32 Previous studies have found the IFN production blockade via antagonism of type I and type III IFN responses in several SARS‐CoV patients33, 34, 35, 36 and comparable escape strategies have also been described in SARS‐CoV‐2 infection.37 Patients with severe COVID‐19 are characterized by suppressed type I and III IFNs.38, 39 In vitro, SARS‐CoV‐2 infection presents an increased susceptibility to type I IFN compared to SARS‐CoV.37
Studies have focused on the importance of the IFN pathway in relation to the clinical severity and treatment options of COVID‐19.38, 39 Zhang et al40 investigated the contribution of genetic variants of genes encoding molecules involved in the IFN pathway and found that patients with life‐threatening COVID‐19 carried rare variants in 13 loci that lead to loss‐of function and therefore impaired IFN pathway. Bastard et al41 also found an impaired Type I IFN signaling pathway in 10% of adult patients with life‐threatening SARS‐CoV‐2 infection as they had autoantibodies against Type I IFNs, and especially against IFN‐α and ΙFN‐ω, which lead to the inability of the immune system to fight the infection.
Stimulation of peripheral proinflammatory cytokines, neutrophils, and monocytes/macrophages in the lower respiratory tract has been reported in symptomatic COVID‐19 patients, but the role of dendritic cells in COVID‐19 remains elusive.42, 43 Strong innate immune responses are in a positive feedback loop with proinflammatory cytokines. This cytokine cascade is associated with increased severity rates in adults not only on SARS‐CoV‐2, but also on SARS and MERS.44 Despite the limited spread of SARS and MERS compared to SARS‐CoV‐2, only few cases of severe clinical manifestations in children and adolescents have been reported.45 SARS is a relatively mild respiratory illness in young children and most common clinical manifestations included cough, chills, fatigue, vomiting, diarrhea, rhinorrhea, diarrhea, and respiratory distress.45 There are 14 pediatric patients with confirmed MERS‐CoV infection, which accounts for 2% of total MERS‐CoV reported cases.45 However, only two of them died: a 2‐year‐old child with cystic fibrosis46 and a 9‐month‐old with congenital nephrotic syndrome.47
In a large‐scale study from China, a correlation between disease severity and elements of innate immunity was investigated. For this purpose, 182 children (≤16 years old) positive for SARS‐CoV‐2 were recruited. An increase in serum procalcitonin (PCT), IL‐2, IL‐4, IL‐6, IL‐10, TNF‐α as well as a decrease in complement C3 was revealed in children with SARS‐CoV‐2 pneumonia.48, 49
Pediatric patients are characterized by robust innate immune responses due to the high production of NK cells compared to older patients.50 In children, NK cells produce IL‐17A in the early stages of the infection, a cytokine which plays an immunological protective role in the development of lung disease.51 Gruber et al22 investigated the immune profiles of 9 MIS‐C patients and found reduced numbers of NK lymphocyte subsets in peripheral blood, mainly due to extravasation and chemotaxis processes to affected tissues. NK cells recruitment was stimulated by many cytokines and chemokines, including CCL19, CXCL10, and CDCP1.22
4. Humoral immunity
After SARS‐CoV‐2 infection, the majority of patients develop detectable serum antibodies against the receptor‐binding domain (RBD) of the viral S protein with neutralizing and non‐neutralizing activity.52, 53 Antibodies produced by B‐ and T‐cell immune mechanisms recognize SARS‐CoV‐2 antigen epitopes beginning 4–8 days after the onset of COVID‐19 symptoms. These responses consist of an initial immunoglobulin M (IgM) antibody production, followed by IgA and IgG increase in body circulation.54 IgG antibodies are usually detectable 16–20 days after infection in approximately all patients.55, 56 In a recent study in which 188 COVID‐19 patients aged 19–81 years were included, Dan et al57 showed that all individuals had detectable SARS‐CoV‐2 anti‐S and anti‐RBD IgG antibodies in serum approximately 5 to 8 months post symptom onset. Rodda et al58 showed the persistence of memory B‐cells and T‐cells for at least 3 months and the increase of SARS‐CoV‐2‐specific IgG+ RBD‐specific memory B cells over time in patients who recovered from mild COVID‐19.
Until recently, most seroepidemiological studies refer to adult patients that are mainly in the acute phase of the infection. For this reason, the exact duration of SARS‐CoV‐2 antibody response in children requires further investigation. This duration probably depends on the severity of the infection and the viral load to which the individual was exposed.59 There are indications that support the presence of humoral responses 3–5 months after the onset of symptoms.60, 61, 62 Recent studies showed that less than 50% of adult patients developed neutralizing antibodies (NAbs) in the acute phase of infection with median time 36 days, but all of them had detectable antibodies in the convalescent phase. There are studies indicating that NAbs production is largely dependent on the exposure to viral load and the severity of the disease.63 The duration of NAbs as investigated in mild symptomatic adult patients, which was estimated to last at least 3 months after the onset of symptoms.64
Children often experience robust antibody production within the first 3 weeks post infection and an estimated seroconversion time to IgG antibodies in the first week. Additionally, there is an increase in IgG specific B‐cell rates in children with SARS‐CoV‐2, indicating a rapid and effective humoral immune response.65, 66 In a seroprevalence pediatric study (n = 208), children aged 10–16 years have 2–3 times higher positive antibody titers than children under 10 years old.67 Possible etiologies that enhance the innate immune response and humoral activity constitute a) the decreased expression of the ACE2 receptor in the upper respiratory epithelium of younger children and b) the antibody cross‐reactivity with other common cold CoVs, in which older children are more frequently exposed to.67
In a large population study involving approximately 2000 children and adolescents (5–21 years), a seropositivity of 1.35% were detected. Notably, 46.2% of the seropositive children were asymptomatic.68 It was found that the titer of antibodies was low compared to that of the adults, even though 92.3% of children produced antibodies with neutralizing capacity, which may protect them from reinfection. However, the duration of these neutralizing antibodies remains unknown.68
5. Cellular immunity
T‐cell responses play an important role in the elimination of viral infections either by directly neutralizing infected cells or by instrumenting immunological memory.
It has been shown that immunological memory against other coronaviruses, like SARS, is so strong that it can even last for several years.69 Despite the decreased serum antibody levels in patients who recovered from SARS or MERS, cell‐mediated immunity was still present at least 10 years after infection.70 SARS‐CoV‐2 specific CD4+ and CD8+ T‐cells were detected 100% and 70%, respectively, in COVID‐19 convalescent adult patients against various viral epitopes, including spike (S), nucleocapsid (N) and membrane (M) proteins.71 Detection of SARS‐CoV‐2 reactive CD4+ T‐cell responses in unexposed individuals suggests a cross‐reactive recognition of common antigen epitopes between SARS‐CoV‐2 and other CoVs.72, 73
SARS‐CoV‐2 does not cause lymphopenia only by directly inducing apoptosis of lymphocytes, thymus suppression, and bone marrow impairment mechanisms, but also due to redistribution of T lymphocytes in affected organs via peripheral blood circulation.74 In patients with moderate and severe COVID‐19, migration of dendritic cells, monocytes, and lymphocytes from peripheric tissues to the lower respiratory system was associated with increased inflammatory markers, augmented intensity of radiographic findings in the lungs and poor clinical outcome of the disease.75, 76
In contrast to other viruses, the SARS‐CoV‐2 virus suppresses T‐cell activity via induction of severe lymphopenia and exhaustion of T cells, suggesting an important impairment in the immunoregulatory arm of the adaptive immune response.77 Adults with severe SARS‐CoV‐2 infection usually present with decreased lymphocyte counts, including total numbers of CD4+, CD8+, and T regulatory cells, being indicative of the poor disease outcome.78, 79
In a cohort study of 452 patients with laboratory‐confirmed COVID‐19 and in which 50% of them presented with severe disease, Qin et al reported a statistically significant (P‐value 0.04) decreases of (CD3+, CD4+, CD25+, CD127low+) T regulatory cells (3.7/μl vs. 4.5/μl) in severe adult COVID‐19 cases in Wuhan.78 Chen et al80 showed that the CD45RA+ T‐regulatory cell frequencies were 0.5% in severe and 1.1% in moderate COVID‐19 cases with a P‐value of 0.02, but the total number of Treg cells did not have statistically significant differences (4.7% vs. 3.9%; P‐value 0.92). Consequently, the correlation between the number of Treg cells and COVID‐19 requires further investigation.
Lymphopenia is a marker of severe disease in children with COVID‐19 as well, even though it is rarely encountered. Kosmeri et al81 suggest that lymphopenia is infrequently documented in children possibly due to the immature immune system and decreased ACE2 expression compared to adults. In a recent meta‐analysis, lymphocytosis and leukopenia were the most common laboratory abnormalities, encountered in 22% and 21% of hospitalized pediatric patients respectively.82 In contrast to adults who mainly express Th1, CD25+ and IFN‐γ inflammatory response, children are characterized by Th2 and Th17 immune responses.51, 83 Although children are frequently exposed to CoVs, immunological memory and neutralizing antibody production against SARS‐CoV‐2 are limited due to their reduced lifetime exposure to antigens.84
6. Multisystem inflammatory syndrome in children
In April 2020, several reports from Europe, Canada, and the USA described a rare, novel clinical phenotype that shared similar clinical characteristics with incomplete Kawasaki syndrome (KS) or TSS. This condition was named MIS‐C and was regarded as a severe complication of COVID‐19 in children.15 Even though many of these patients meet the criteria for complete or incomplete Kawasaki syndrome (KS) or TSS. This condition was named MIS‐C and was regarded as a severe complication of COVID‐19 in children.15 Even though many of these patients meet the criteria for complete or incomplete KD, there are many differences among epidemiological aspects, including age of onset and ethnicity.85 KD, there are many differences among epidemiological aspects, including age of onset and ethnicity.85
Notably, many affected children did not have positive reverse transcription polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 at the moment of MIS‐C diagnosis, but they had positive seroimmunology testing.86 These findings support the hypothesis of a late‐onset abnormal immune response that occurs days or weeks after acute infection. Comparable deregulated immunophenotype and immune responses have previously been described in KD, macrophage activation syndrome (MAS), and cytokine release syndrome.87 Immune responses that occur in MIS‐C differ from KD and MAS, but the exact mechanisms stimulated by SARS‐CoV‐2 infection remain unknown.88, 89 Notwithstanding, recent data support that an increase in IL‐17 as well as T‐cell activation can distinguish patients with KD from those with MIS‐C.90
A recent study described the immunological differences among the pediatric population with MIS‐C and SARS‐CoV‐2 positive adults presenting with mild disease and acute respiratory distress syndrome (ARDS).90 In contrast to adults, children with MIS‐C produce predominantly anti‐S IgG but low amounts of anti‐N IgG antibodies.90 Compared to adults with ARDS, children with MIS‐C expressed a lower number of antibodies accompanied by less neutralizing activity.90 Consequently, significantly lower titers of anti‐N IgG and neutralizing activity were identified in children compared to adults regardless of the severity, such as MIS‐C.90 There was also a positive correlation between anti‐S‐RBD IgG and the onset of clinical manifestations and a negative correlation between age and neutralizing activity in children without MIS‐C.90, 91, 92
In a study, which included 127 children with pneumonia, an increase of IL‐10 and decreased levels of CD4+ CD25+ T lymphocytes, NK and CD4+/CD8+ T cell ratios were detected.93 Patients with MIS‐C had elevated levels of IL‐6, TNF‐α, and IFN gamma‐induced protein 10 (IP‐10) in serum as well as enhanced antibody‐dependent cellular phagocytosis (ADCP) activity.51 The increase of IL‐17A, produced by CD4+ T cells, CD8+ T cells, gamma‐delta T cells, invariant NK T‐cells, innate lymphoid cells and neutrophils, implies a possible protective role in the development of lung disease.51
Peripheral immunophenotyping performed in children with MIS‐C showed significant neutrophilia, lymphopenia with T‐cell exhaustion, and elevation of cytokines, including IL‐1β, IL‐6, IL‐8, IL‐10, IL‐17 and IFN‐γ, which were present only in the acute phase of MIS‐C.94 Additionally, compared to children with severe COVID‐19, children with MIS‐C have a higher proportion of TNF‐α and IL‐10.95
7. Why is COVID‐19 less severe in children?
The rapid progression of COVID‐19 pandemic has attracted the interest of the scientific community worldwide. The majority of patients that require hospitalization are elderly with comorbidities and fatal COVID‐19 case rates are remarkably lower in pediatric populations. Several hypotheses have been proposed to explain those age‐related differences in disease severity.
SARS‐CoV‐2 enters the human body mainly through ACE2 receptor and transmembrane protease serine 2 (TMPRSS2) in the nasopharyngeal cells.96, 97 Disease severity, as well as COVID‐19 specific symptoms such as loss of taste and smell, probably depends on ACE2 and TMPRSS2 quantitative expression in the respiratory tract, renal, gastrointestinal and cardiovascular systems.96, 97 This age‐dependent expression is significantly lower in children compared to adults (Table 1).98
TABLE 1.
Factors involved in age‐related differences of SARS‐CoV‐2 pathogenesis
| Factors involved in age‐related differences of SARS‐CoV‐2 pathogenesis | Adults | Children | References |
|---|---|---|---|
| Expression of ACE2 receptor and TMPRSS2 | ↑ | ↓ | 96, 98, 136 |
| Comorbidities and medications | ↑ | ↓ | 99, 100, 101, 102 |
| Innate immune responses | ↓ | ↑ | 103 |
| Adaptive immune responses | ↑ | ↓ | 72, 109 |
| Pre‐existing antibodies to other HCoVs | ↑ | ↓ | 104, 109 |
| Trained immune responses | ↓ | ↑ | 125, 126 |
| ADE and macrophage hyperactivation | ↑ | ↓ | 106, 117, 119 |
| Cytokine storm | usually ↑ | usually ↓(except for MIS‐C) | 80, 121, 122 |
| Vitamin D and other micronutrients | ↓ | ↑ | 127, 130 |
SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin‐converting enzyme 2; TMPRSS2, transmembrane protease serine 2; HCoVs, human coronaviruses; ADE, antibody‐dependent enhancement; MIS‐C, multisystem inflammatory syndrome in children.
Pediatric patients have less travelling, hospitalization and workplace exposure rates, as well as fewer pulmonary and extrapulmonary comorbidities that are associated with severe COVID‐19 in adulthood.11, 99 Comorbidities include chronic obstructive pulmonary disease, endothelial injury, hypercoagulopathy, heart failure, hypertension, diabetes, obesity, malignancy, chronic kidney disease or medications that are increase the risk of the severity of illness, such as ACE inhibitors or angiotensin II receptor blockers (ARBs).100, 101, 102
Older adults are characterized by reduced innate and adaptive immune responses that result in decreased viral clearance.72, 103 Previous exposure to other HCoVs, mainly those that cause the common cold, leads to pre‐existing cross‐reactive specific viral T‐cell responses, even in individuals who have never been exposed to SARS‐CoV‐2 before.71, 72, 104 The higher proportion of memory cells in adults and the absence of naive T cells, which are abundant in young children, may potentially contribute to the massive T cell‐derived cytokine release mainly observed in adults with ARDS. Although children are frequently exposed to human coronaviruses, their T‐cell responses and neutralizing activity are lower compared to adults.105
There are many mechanisms that are usually encountered in adults and affect the immune responses of the host, including antibody‐dependent enhancement (ADE), macrophage hyperstimulation, and cytokine storm. ADE is a mechanism usually promoted by viral infections or vaccination,106 such as dengue virus, SARS‐CoV, and MERS‐CoV vaccination efforts,107, 108 in which neutralizing antibody production is stimulated by previously circulating viral particles of serotypes.109, 110
Korber et al111 described a specific mutation in the RBD of SARS‐CoV‐2 S1 subunit (D614G), which is rapidly progressing worldwide. Beretta et al112 suggested that a possible effect of this mutation is a potential inducer of ADE, since it shares a common linear epitope of the SARS‐CoV spike which is located close to RBD and might influence the interaction between RBD and ACE2 receptor. Even though Zhou et al113 described the presence of anti‐RBD antibodies that induce ADE of viral entry in Raji cells through the Fcγ receptor‐dependent mechanism, there are also some RBD epitopes that were associated with only neutralizing activity in the absence of ADE effect. These data suggest that there are different, non‐overlapping RBD epitopes regarding neutralization and ADE.113 Notably, a recent case report of a 25 year‐old man with symptomatic SARS‐CoV‐2 reinfection might be attributed to ADE mechanism.114
An explanation for ADE after exposure to SARS‐CoV‐2 was hypothesized by Zimmermann et al, proposing the a possible lifetime exposure to HCoVs makes the elderly vulnerable to ADE, due to the high levels of non‐neutralizing cross‐reactive antibodies, resulting in severe clinical manifestations and poor outcome of the disease, compared to children that are characterized by a lower level of pre‐existing non‐neutralizing antibodies.115
Until recently, there are no specific data that demonstrate ADE in children. However, a model of MIS‐C has been proposed by Ricke et al in which ADE activation of mast cells in children with COVID‐19 is hypothesized.116 Degranulation of mast cells with Fc receptor‐bound SARS‐CoV‐2 antibodies leads to an hyperinflammatory response, which results in increased histamine levels, upregulated prostaglandin E2 (PGE2), leading to increased risks of coronary artery aneurysms.116
In conjunction with immune complex formation by binding to IgG Fcγ receptors,117 macrophages are hyperactivated and clinical manifestations of the disease become more severe as a result of inflammatory response exacerbation. Monocytes differentiate to macrophages at the site of inflammation after monocyte chemoattractant protein‐1 (MCP‐1) exposure.118 It seems possible that adults have decreased L‐selectin and increased CD11b,119, 120 both of which result in irregular macrophage migration.
Cytokine storm, defined as the abnormal systemic immune reaction associated with increased levels of inflammatory cytokines and activation of T lymphocytes and macrophages. This deregulated cytokine release of uncontrolled systemic inflammatory response results in elevated IL‐1β, IL‐2, IL‐6, IL‐7, IL‐8, IL‐10, granulocyte colony stimulating factor (GCSF), MCP‐1, TNF‐α with multiple organ dysfunction, such as MIS‐C (Table 1).80, 121, 122
Recently, it is obvious that cells of the innate immune system can be trained by past infections, vaccinations, or microbial components to enhance immune responses to future triggers.123, 124 This effect is known as “trained immunity”, which has been shown after bacille Calmette‐Guérin (BCG), measles and oral polio vaccination or certain infections.123, 125 Given that there are children who have never been immunized against tuberculosis, trained immunity may not be explained by BCG vaccination. Zimmermann et al115 make the hypothesis that since the majority of vaccinations are implemented early in childhood, trained immunity mechanisms may contribute to a more efficient inhibition of viral replication and explain the age‐related differences among COVID‐19 clinical spectrum. However, their role in SARS‐CoV‐2 infection still requires further investigation, especially after the first vaccination.126
Deficiencies of certain micronutrients, such as vitamin D, zinc, and selenium have been associated with more severe disease in observational studies.127, 128 Vitamin D has a significant role in the prevention of viral infections by suppressing their replication via various immunoregulatory pathways.129 Even though there is growing interest in the role of vitamin D in the immune response during SARS‐CoV‐2 infection, no clear evidence that vitamin D supplementation reduces the risk or severity of COVID‐19 has been established. Vitamin D levels are usually lower in elderly, due to inadequate supplementation.130 However, vitamin D is often routinely supplemented to infants younger than 1 year in many countries.115
In a randomized trial from Brazil, 244 patients with moderate COVID‐19 disease were divided in two groups, evaluating the effect of a single high dose of vitamin D3 versus placebo.131 Even though admission to the intensive care unit (ICU) and need for mechanical ventilation were both decreased 16.0% vs. 21.2% and 7.6% vs. 14.4%, respectively, these differences were not statistically significant (P‐values 0.3 and 0.09 respectively). 131 However, it is notable that Lau et al observed in a population of 20 patients with COVID‐19 that 84% of COVID‐19 patients in ICU had vitamin D deficiency compared to 57.1% of non‐ICU hospitalized COVID‐19 patients.132
A retrospective study showed that children with COVID‐19 had lower vitamin‐D titers compared to children without COVID‐19.133 Among children with SARS‐CoV‐2 infection, there was a negative correlation between vitamin D levels and fever as well.133 A recent review suggests that vitamin D levels could be used as a predictive biomarker of MIS‐C and suggests that correction of abnormal vitamin D levels in severe MIS‐C may favorably affect the outcome of the disease.134
8. Questions to be answered
The paramount interest of scientific investigation focuses on the severe forms of COVID‐19 disease, most of which are predominantly occurring in the elderly with certain underlying medical conditions. However, the surprisingly effective immune responses against SARS‐CoV‐2 in children raise reasonable questions for future research in both innate and adaptive arms of immunity. Notably, there are significant differences in clinical severity depending on gender, age, ethnicity, or comorbidities, but the exact mechanisms are only partially understood.
The role of the innate immune response in effective viral clearance in the early stages of the disease in children requires further investigation to identify certain measurable immunological biomarkers that are predictive of the severity of the disease, of the response to treatment and convalescence. The different clinical manifestations of the disease do not reflect the exact contribution degree of humoral and cellular immune responses, neither the effective elimination of infection, nor the protection of reinfection, a condition already described in adult patients.
Since the beginning of SARS‐CoV‐2 pandemic, which is now more than 12 months, limited data have been published regarding the exact duration of antibody production, antibody kinetics, T‐cell memory, and the factors that contribute to T‐cell activation or exhaustion in children. Although non‐neutralizing antibody production has been described well in children with COVID‐19 with a wide range of clinical manifestations, the exact percentage and duration of neutralizing remain questionable. It also needs to be clarified the immunological mechanisms that contribute to MIS‐C by enlightening the key role of T‐regulatory cells, which assist in the establishment of protective immunity and immunological homeostasis.
9. Conclusion
This review summarizes the latest knowledge of the innate and adaptive immune responses against SARS‐CoV‐2 in both children and adults. The decryption of the immune mechanisms activated by SARS‐CoV‐2 infection as well as the detection of prognostic immuno‐biomarkers related to the severity of the disease, age and sex will contribute to confrontation or prevent severe clinical manifestations or re infect ion. Children are an important group in understanding these mechanisms as the majority of them are asymptomatic or mild symptomatic. Therefore, so far, there is limited knowledge regarding the immune responses in children and further research is required.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Filippatos F, Tatsi E‐B, Michos A. Immune response to SARS‐CoV‐2 in children: A review of the current knowledge. Pediatr Invest. 2021;5:217‐228. 10.1002/ped4.12283
REFERENCES
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, et al. Bats are natural reservoirs of SARS‐like coronaviruses. Science. 2005;310:676‐679. [DOI] [PubMed] [Google Scholar]
- 3.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. 2018;100:163‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabaan AA, Al‐Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS, et al. SARS‐CoV‐2, SARS‐CoV, and MERS‐COV: A comparative overview. Infez Med. 2020;28:174‐184. [PubMed] [Google Scholar]
- 6.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posfay‐Barbe KM, Wagner N, Gauthey M, Moussaoui D, Loevy N, Diana A, et al. COVID‐19 in children and the dynamics of infection in families. Pediatrics. 2020;146:e20201576. [DOI] [PubMed] [Google Scholar]
- 8.Götzinger F, Santiago‐García B, Noguera‐Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macartney K, Quinn HE, Pillsbury AJ, Koirala A, Deng L, Winkler N, et al. Transmission of SARS‐CoV‐2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;4:807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown NE, Bryant‐Genevier J, Bandy U, Browning CA, Berns AL, Dott M, et al. Antibody Responses after Classroom Exposure to Teacher with Coronavirus Disease, March 2020. Emerg Infect Dis. 2020;26:2263‐2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 13.Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID‐19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustafa NM, A Selim L. Characterisation of COVID‐19 pandemic in paediatric age group: A systematic review and meta‐analysis. J Clin Virol. 2020;128:104395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son M, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal immunity in COVID‐19: A neglected but critical aspect of SARS‐CoV‐2 infection. Front Immunol. 2020;11:611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breedveld A, van Egmond M. IgA and FcαRI: Pathological roles and therapeutic opportunities. Front Immunol. 2019;10:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zohar T, Alter G. Dissecting antibody‐mediated protection against SARS‐CoV‐2. Nat Rev Immunol. 2020;20:392‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervia C, Nilsson J, Zurbuchen Y, Valaperti A, Schreiner J, Wolfensberger A, et al. Systemic and mucosal antibody responses specific to SARS‐CoV‐2 during mild versus severe COVID‐19. J Allergy Clin Immunol. 2021;147:545‐557.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weemaes C, Klasen I, Göertz J, Beldhuis‐Valkis M, Olafsson O, Haraldsson A. Development of immunoglobulin A in infancy and childhood. Scand J Immunol. 2003;58:642‐648. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Ravichandran S, Lee Y, Grubbs G, Coyle EM, Klenow L, et al. Antibody affinity maturation and plasma IgA associate with clinical outcome in hospitalized COVID‐19 patients. Nat Commun. 2021;12:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS‐C). Cell. 2020;183:982‐995.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tschernig T, Pabst R. Bronchus‐associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68:1‐8. [DOI] [PubMed] [Google Scholar]
- 24.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783‐801. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805‐820. [DOI] [PubMed] [Google Scholar]
- 26.Thompson MR, Kaminski JJ, Kurt‐Jones EA, Fitzgerald KA. Pattern recognition receptors and the innate immune response to viral infection. Viruses. 2011;3:920‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkins C, Gale M Jr. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanmohammadi S, Rezaei N. Role of Toll‐like receptors in the pathogenesis of COVID‐19. J Med Virol. 2021;93:2735‐2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS‐CoV‐2 spike glycoprotein with ACE‐2 receptor homologs and human TLRs. J Med Virol. 2020;92:2105‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hensley LE, Fritz EA, Jahrling PB, Karp CL, Huggins JW, Geisbert TW. Interferon‐beta 1a and SARS Coronavirus Replication. Emerg Infect Dis. 2004;10:317‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clementi N, Ferrarese R, Criscuolo E, Diotti RA, Castelli M, Scagnolari C, et al. Interferon‐β‐1a inhibition of severe acute respiratory syndrome‐coronavirus 2 in vitro when administered after virus infection. J Infect Dis. 2020;222:722‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amor S, Fernández Blanco L, Baker D. Innate immunity during SARS‐CoV‐2: evasion strategies and activation trigger hypoxia and vascular damage. Clin Exp Immunol. 2020;202:193‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wathelet MG, Orr M, Frieman MB, Baric RS. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J Virol. 2007;81:11620‐11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minakshi R, Padhan K, Rani M, Khan N, Ahmad F, Jameel S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand‐independent downregulation of the type 1 interferon receptor. PLoS One. 2009;4:e8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siu KL, Kok KH, Ng MJ, Poon V, Yuen KY, Zheng BJ, et al. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J Biol Chem. 2009;284:16202‐16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron MJ, Ran L, Xu L, Danesh A, Bermejo‐Martin JF, Cameron CM, et al. Interferon‐mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692‐8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181:1036‐1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science. 2020;369:718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin F, Shen K. Type I interferon: From innate response to treatment for COVID‐19. Pediatr Investig. 2020;4:275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada‐Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370:eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single‐cell landscape of bronchoalveolar immune cells in patients with COVID‐19. Nat Med. 2020;26:842‐844. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Du X, Chen J, Jin Y, Peng L, Wang H, et al. Neutrophil‐to‐lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID‐19. J Infect. 2020;81:e6‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Channappanavar R, Perlman S. Pathogenic human corona virus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aleebrahim‐Dehkordi E, Soveyzi F, Deravi N, Rabbani Z, Saghazadeh A, Rezaei N. Human coronaviruses SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2 in children. J Pediatr Nurs. 2021;56:70‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Memish ZA, Al‐Tawfiq JA, Assiri A, AlRabiah FA, Al Hajjar S, Albarrak A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33:904‐906. [DOI] [PubMed] [Google Scholar]
- 47.Thabet F, Chehab M, Bafaqih H, AlMohaimeed S. Middle East respiratory syndrome coronavirus in children. Saudi Med J. 2015;36:484‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du H, Dong X, Zhang JJ, Cao YY, Akdis M, Huang PQ, et al. Clinical characteristics of 182 pediatric COVID‐19 patients with different severities and allergic status. Allergy. 2021;76:510‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw AC, Goldstein DR, Montgomery RR. Age‐dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pierce CA, Preston‐Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, et al. Immune responses to SARS‐CoV‐2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 53.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang AT, Garcia‐Carreras B, Hitchings M, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71:2688‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caturegli G, Materi J, Howard BM, Caturegli P. Clinical validity of serum antibodies to SARS‐CoV‐2: A case‐Control study. Ann Intern Med. 2020;173:614‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell. 2021;184:169‐83.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody Responses to SARS‐CoV‐2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2027‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383:1724‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS‐CoV‐2 spike protein in COVID‐19 patients. Sci Immunol. 2020;5:eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti‐SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383:1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu P, Cai J, Jia R, Xia S, Wang X, Cao L, et al. Dynamic surveillance of SARS‐CoV‐2 shedding and neutralizing antibody in children with COVID‐19. Emerg Microbes Infect. 2020;9:1254‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell. 2021;184:169‐83.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Xu J, Jia R, Yi C, Gu W, Liu P, et al. Protective humoral immunity in SARS‐CoV‐2 infected pediatric patients. Cell Mol Immunol. 2020;17:768‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang R, Jin F, Cao S, Yuan H, Qu J, Zhang J, et al. Seroprevalence of SARS‐CoV‐2 infections among children visiting a hospital. Pediatr Investig. 2020;4:236‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Posfay‐Barbe KM, Andrey DO, Virzi J, Cohen P, Pigny F, Goncalves AR, et al. Prevalence of immunoglobulin G (IgG) against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and evaluation of a rapid MEDsan IgG test in children seeking medical care. Clin Infect Dis. 2020;72:e192‐e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szépfalusi Z, Schmidthaler K, Sieber J, Kopanja S, Götzinger F, Schoof A, et al. Lessons from low seroprevalence of SARS‐CoV‐2 antibodies in schoolchildren: A cross‐sectional study. Pediatr Allergy Immunol. 2021; 10.1111/pai.13459. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Bert N, Tan AT, Kunasegaran K, Tham C, Hafezi M, Chia A, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020;584:457‐462. [DOI] [PubMed] [Google Scholar]
- 70.Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: Comparison with SARS and MERS. Rev Med Virol. 2020;30:e2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS‐CoV‐2 Coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020;181:1489‐501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, et al. SARS‐CoV‐2‐reactive T cells in healthy donors and patients with COVID‐19. Nature. 2020;587:270‐274. [DOI] [PubMed] [Google Scholar]
- 73.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jafarzadeh A, Jafarzadeh S, Nozari P, Mokhtari P, Nemati M. Lymphopenia an important immunological abnormality in patients with COVID‐19: Possible mechanisms. Scand J Immunol. 2021;93:e12967. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez‐Cerrillo I, Landete P, Aldave B, Sánchez‐Alonso S, Sánchez‐Azofra A, Marcos‐Jiménez A, et al. COVID‐19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes. J Clin Invest. 2020;130:6290‐6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kvedaraite E, Hertwig L, Sinha I, Ponzetta A, Hed Myrberg I, Lourda M, et al. Major alterations in the mononuclear phagocyte landscape associated with COVID‐19 severity. Proc Natl Acad Sci U S A. 2021;118:e2018587118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Gui J. Cell‐mediated immunity to SARS‐CoV‐2. Pediatr Investig. 2020;4:281‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369:77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kosmeri C, Koumpis E, Tsabouri S, Siomou E, Makis A. Hematological manifestations of SARS‐CoV‐2 in children. Pediatr Blood Cancer. 2020;67:e28745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y. The clinical characteristics of pediatric inpatients with SARS‐CoV‐2 infection: A meta‐analysis and systematic review. J Med Virol. 2021;93:234‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peron JPS, Nakaya H. Susceptibility of the elderly to SARS‐CoV‐2 infection: ACE‐2 overexpression, shedding, and antibody‐dependent enhancement (ADE). Clinics (Sao Paulo). 2020;75:e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324:259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS‐CoV‐2 infection. Nat Med. 2020;26:1701‐1707. [DOI] [PubMed] [Google Scholar]
- 87.Lee PY, Day‐Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, et al. Distinct clinical and immunological features of SARS‐CoV‐2‐induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942‐5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weisberg SP, Connors T, Zhu Y, Baldwin M, Lin WH, Wontakal S, et al. Antibody responses to SARS‐CoV2 are distinct in children with MIS‐C compared to adults with COVID‐19. medRxiv. 2020;2020.07.12.20151068. (Preprint) [Google Scholar]
- 89.Feng Z, Bao Y, Yang Y, Zheng Y, Shen K. Severe acute respiratory syndrome coronavirus 2‐induced multisystem inflammatory syndrome in children. Pediatr Investig. 2020;4:257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, et al. Distinct antibody responses to SARS‐CoV‐2 in children and adults across the COVID‐19 clinical spectrum. Nat Immunol. 2021;22:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson EM, Diorio C, Goodwin EC, McNerney KO, Weirick ME, Gouma S, et al. SARS‐CoV‐2 antibody responses in children with MIS‐C and mild and severe COVID‐19. medRxiv. 2020;2020.08.17.20176552. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rostad CA, Chahroudi A, Mantus G, Lapp SA, Teherani M, Macoy L, et al. Quantitative SARS‐CoV‐2 serology in children with multisystem inflammatory syndrome (MIS‐C). Pediatrics. 2020;146:e2020018242. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Deng W, Xiong H, Li H, Chen Z, Nie Y, et al. Immune‐related factors associated with pneumonia in 127 children with coronavirus disease 2019 in Wuhan. Pediatr Pulmonol. 2020;55:2354‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS‐CoV‐2 infection. Nat Med. 2020;26:1701‐1707. [DOI] [PubMed] [Google Scholar]
- 95.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, et al. Multisystem inflammatory syndrome in children and COVID‐19 are distinct presentations of SARS‐CoV‐2. J Clin Invest. 2020;130:5967‐5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xydakis MS, Dehgani‐Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, et al. Smell and taste dysfunction in patients with COVID‐19. Lancet Infect Dis. 2020;20:1015‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schuler BA, Habermann AC, Plosa EJ, Taylor CJ, Jetter C, Negretti NM, et al. Age‐determined expression of priming protease TMPRSS2 and localization of SARS‐CoV‐2 in lung epithelium. J Clin Invest. 2021;131:e140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mannheim J, Gretsch S, Layden JE, Fricchione MJ. Characteristics of hospitalized pediatric coronavirus disease 2019 cases in Chicago, Illinois, March‐April 2020. J Pediatric Infect Dis Soc. 2020;9:519‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐Angiotensin‐Aldosterone System Blockers and the Risk of Covid‐19. N Engl J Med. 2020;382:2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the innate immune system: An update. Curr Immunol Rev. 2011;7:104‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sette A, Crotty S. Pre‐existing immunity to SARS‐CoV‐2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pierce CA, Preston‐Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, et al. Immune responses to SARS‐CoV‐2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tirado SM, Yoon KJ. Antibody‐dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69‐86. [DOI] [PubMed] [Google Scholar]
- 107.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327‐340. [DOI] [PubMed] [Google Scholar]
- 108.Hashem AM, Algaissi A, Agrawal AS, Al‐Amri SS, Alhabbab RY, Sohrab SS, et al. A highly immunogenic, protective, and safe adenovirus‐based vaccine expressing Middle East respiratory syndrome coronavirus S1‐CD40L fusion protein in a transgenic human dipeptidyl peptidase 4 mouse model. J Infect Dis. 2019;220:1558‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lv H, Wu NC, Tsang OT, Yuan M, Perera R, Leung WS, et al. Cross‐reactive antibody response between SARS‐CoV‐2 and SARS‐CoV infections. Cell Rep. 2020;31:107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, et al. Molecular mechanism for antibody‐dependent enhancement of coronavirus entry. J Virol. 2020;94:e02015‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS‐CoV‐2. bioRxiv. 2020. 10.1101/2020.04.29.069054. [DOI] [Google Scholar]
- 112.Beretta A, Cranage M, Zipeto D. Is cross‐reactive immunity triggering COVID‐19 immunopathogenesis? Front Immunol. 2020;11:567710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Y, Liu Z, Li S, Xu W, Zhang Q, Silva IT, et al. Enhancement versus neutralization by SARS‐CoV‐2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 2021;34:108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A, et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis. 2021;21:52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zimmermann P, Curtis N. Why is COVID‐19 less severe in children? A review of the proposed mechanisms underlying the age‐related difference in severity of SARS‐CoV‐2 infections. Arch Dis Child. 2020:archdischild‐2020‐320338. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 116.Ricke DO, Gherlone N, Fremont‐Smith P, Tisdall P, Fremont‐Smith M. Kawasaki disease, multisystem inflammatory syndrome in children: Antibody‐induced mast cell activation hypothesis. J Pediatrics Pediatr Med. 2020;4:1‐7. [Google Scholar]
- 117.Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody‐dependent enhancement. Nat Rev Immunol. 2020;20:633‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gschwandtner M, Derler R, Midwood KS. More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol. 2019;10:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.DeMartinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82:415‐420. [DOI] [PubMed] [Google Scholar]
- 120.van Royen N, Hoefer I, Böttinger M, Hua J, Grundmann S, Voskuil M, et al. Local monocyte chemoattractant protein‐1 therapy increases collateral artery formation in apolipoprotein E‐deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92:218‐225. [DOI] [PubMed] [Google Scholar]
- 121.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID‐19. Cell. 2020;183:968‐81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Netea MG, Domínguez‐Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kerboua KE. The perplexing question of trained immunity vs adaptive memory in COVID‐19. J Med Virol. 2020;92:1858‐1863. [DOI] [PubMed] [Google Scholar]
- 125.Netea MG, Giamarellos‐Bourboulis EJ, Domínguez‐Andrés J, Curtis N, van Crevel R, van de Veerdonk FL, et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS‐CoV‐2 infection. Cell. 2020;181:969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mantovani A, Netea MG. Trained innate immunity, epigenetics, and Covid‐19. N Engl J Med. 2020;383:1078‐1080. [DOI] [PubMed] [Google Scholar]
- 127.Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti‐viral resistance against progressive COVID‐19. Nutrients. 2020;12:2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, et al. Vitamin D insufficiency as a potential culprit in critical COVID‐19 patients. J Med Virol. 2021;93:733‐740. [DOI] [PubMed] [Google Scholar]
- 129.Ali N. Role of vitamin D in preventing of COVID‐19 infection, progression and severity. J Infect Public Health. 2020;13:1373‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mosekilde L. Vitamin D and the elderly. Clin Endocrinol (Oxf). 2005;62:265‐281. [DOI] [PubMed] [Google Scholar]
- 131.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran C, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID‐19: A randomized clinical trial. JAMA. 2021;325:1053‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, et al. Vitamin D insufficiency is prevalent in severe COVID‐19. medRxiv. 2020. 10.1101/2020.04.24.20075838. [DOI] [Google Scholar]
- 133.Yılmaz K, Şen V. Is vitamin D deficiency a risk factor for COVID‐19 in children. Pediatr Pulmonol. 2020;55:3595‐3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feketea G, Vlacha V, Bocsan IC, Vassilopoulou E, Stanciu LA, Zdrenghea M. Vitamin D in corona virus disease 2019 (COVID‐19) related multisystem inflammatory syndrome in children (MIS‐C). Front Immunol. 2021;12:648546. [DOI] [PMC free article] [PubMed] [Google Scholar]


