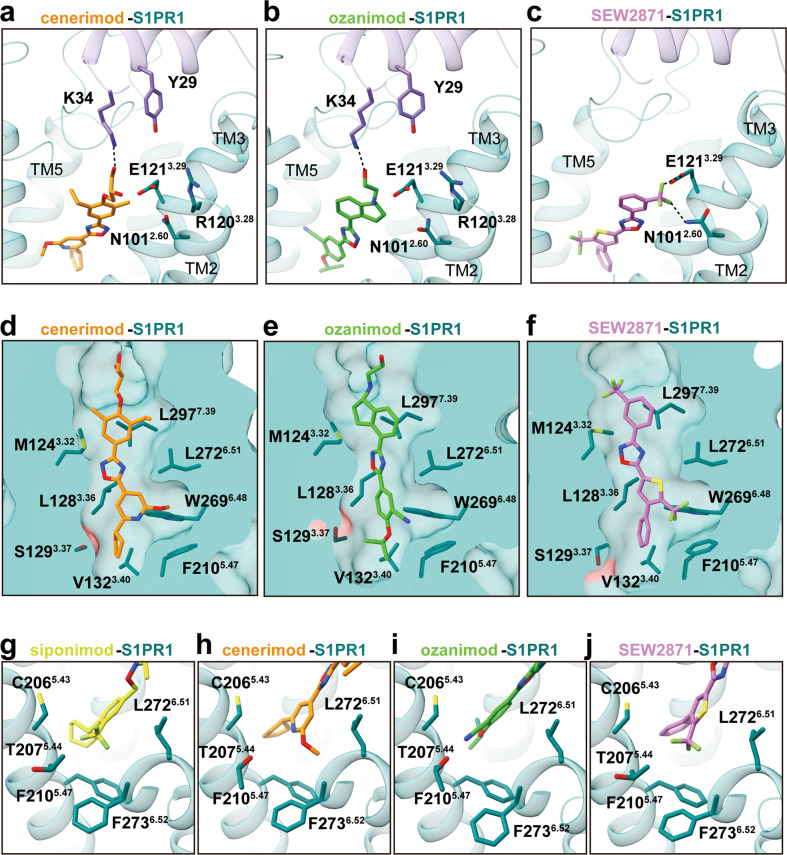
Fig. 3. Comparison of recognition modes of chemically distinct agonists by S1PR1.
a–c Structural basis underlying the recognition of cenerimod (dark orange) with polar-neutral headgroup (a), ozanimod (lime green) with polar-basic headgroup (b), and selective SEW2871 (orchid) without classic polar headgroup (c) by S1PR1. d–f Cut away view of different ligand-binding cavities for cenerimod-bound (d), ozanimod-bound (e), and SEW2871-bound S1PR1 (f). g–j The sub-pocket in the orthosteric site of S1PR, which is composed of the residues C2065.43, T2075.44, F2105.47, L2726.51 and F2736.52 from TM5 and TM6, respectively, accommodates different moieties from four chemically distinct agonists, including trifluoromethyl moiety, methoxy moiety or cyano-moiety.