Abstract
Gout is an inflammatory disease triggered by deposition of monosodium urate (MSU) crystals in the joints, resulting in high neutrophil influx and pain. Here, we studied the role of the inhibitory receptor CD300a in the resolution process in a murine model of gout. We found increased CD300a expression on neutrophils emigrated to the joint. When compared to WT mice, CD300a−/− mice had persistent neutrophil influx till 24 hr after MSU injection. This was associated with increased concentration of IL‐1β and greater tissue damage in the joints of CD300a−/− mice. There was an increase in the percentage of apoptotic neutrophils in the synovial lavage of WT mice, as compared to CD300a−/− mice. This difference was reflected in the decline of efferocytic events in the synovial cavity of CD300a−/− mice 24 hr after MSU injection. A CD300a agonistic antibody was shown, for the first time, to increase apoptosis of human neutrophils, and this was associated with cleavage of caspase‐8. In conclusion, our results reveal an important role of CD300a in the control of leucocyte infiltration, IL‐1β production and caspase‐8 cleavage in neutrophils, contributing to the resolution of inflammation triggered by MSU injection.
Keywords: apoptosis, CD300a, gout, neutrophilic inflammation, resolution of inflammation
Investigation of the role of the immunoreceptor CD300a in the resolution of articular inflammation triggered by MSU crystals in mice. Absence of CD300a delayed resolution of neutrophilic inflammation and activation of CD300a induce neutrophil apoptosis via caspase 8.
Abbreviations
- AD
atopic dermatitis
- AnxV
annexin V
- CD300a− / −
CD300a knockout
- CFSE
carboxyfluorescein succinimidyl ester
- CXCL1
C‐X‐C motif chemokine ligand 1
- ELISA
enzyme‐linked immunosorbent assay
- IL‐1β/5
interleukin‐1β/5;
- ITIM
immunoreceptor tyrosine‐based inhibitory motif
- MSU
monosodium urate
- PE
phosphatidylethanolamine
- PI
propidium iodide
- PS
phosphatidylserine
- SHP‐1
Src homology region 2 domain‐containing phosphatase‐1
- WT
wild type
INTRODUCTION
Gout is the most common cause of acute inflammatory monoarthritis and is characterized by being extremely painful, but self‐limiting. Chronic elevation of serum uric acid levels above the saturation generates monosodium urate (MSU) crystal formation [1]. Deposition of MSU crystals, which occurs predominantly in peripheral joints, triggers an inflammatory process where the NLRP3 inflammasome is activated, resulting in activation of IL‐1β and production of other pro‐inflammatory cytokines [2]. IL‐1β release drives an inflammatory response, which causes vasodilatation and rapid recruitment of neutrophils to the joints, thereby driving the acute inflammatory episode [3]. The presence of neutrophils in the joint plays a key role in the pathogenesis of gout because their activation leads to the release of inflammatory mediators, including IL‐1β, which contribute to pain and tissue damage [4, 5]. In order to avoid these detrimental effects caused by neutrophil activation and to maintain homeostasis, a fine balance between the activating and inhibitory signals of inflammation is necessary.
Negative control of the immune response includes the participation of a diverse variety of inhibitory receptors. The main characteristic of an expanding family of inhibitory receptors is the presence of a consensus amino acid sequence, the immunoreceptor tyrosine‐based inhibitory motif (ITIM), found in their cytoplasmic domain [6]. CD300 is an immune regulatory family that includes activating and inhibitory receptors. Only CD300a and CD300f have ITIMs in the cytoplasmic portion [7]. CD300a is present in myeloid and lymphoid cells of humans and mice [8]. Studies on CD300a demonstrate its capacity to modulate cell activation, cytokine production and cell migration [9, 10, 11]. CD300a was first described in 1999, and relatively little knowledge about it exists to date in several pathologies, which makes its study important [12]. Furthermore, there is strong evidence of the positive selection of the CD300a gene, which suggests a critical role of this receptor on the maintenance of important cellular functions [13, 14].
The counterbalance made by inhibitory receptors, among other mechanisms, is important to avoid an exacerbated inflammatory response, resulting in the elimination of the harmful stimuli, followed by resolution and repair phases [15]. The resolution of inflammation is an active process that aims to restore the affected tissue with a gradual return to homeostasis [16]. While inhibitory receptors act by preventing neutrophil infiltration, in the resolution phase neutrophils become apoptotic, therefore ready to be engulfed by macrophages, which characterizes efferocytosis [15]. Anti‐inflammatory and pro‐resolving actions are not necessarily overlapped, but these actions need to communicate for a better result [17]. Inhibitory receptors are still poorly studied in the context of resolution of inflammation. So far, CD300a has been investigated in the resolution of allergic inflammation. In a model of allergic peritonitis performed on CD300a−/− mice, it has been shown that lack of CD300a results in delayed resolution and eosinophil clearance [18]. Moreover, it has been shown that CD300a expression is increased on eosinophils in lesional skin of patients with atopic dermatitis (AD) and that lack of CD300a results in increased inflammatory cell infiltration and cytokine expression in a murine AD model [19]. Here, we investigate how the immunoreceptor CD300a contributes to resolution in a model of gout induced by injection of MSU crystals in the joint of mice. We demonstrate that in addition to modulating neutrophil influx, CD300a contributes to apoptosis of neutrophils and consequent resolution of inflammation.
METHODS
Mice
Male BALB/c mice (8–12 weeks) were obtained from the Center of bioterism of Universidade Federal de Minas Gerais (UFMG) Brazil. C;129S5 Cd300atm1Lex/Mmucd mice were generated at MMRC, UC Davis, University of California, Davis, CA; they were originally donated by Genentech and backcrossed to BALB/c mice for 10 generations at the Hebrew University of Jerusalem (Jerusalem, Israel). CD300a knockout mice were brought to Brazil and maintained in the animal facilities at Universidade Federal de Minas Gerais (UFMG). All mice were supplied with water and food ad libitum. Mice were maintained in pathogen‐free conditions. This study was carried out in accordance with the recommendations of the law nº 11·794 from National Council for Control of Animal Experimentation – CONCEA, Brazil. The protocol was approved by the Animal Ethics Council – CEUA – at Universidade Federal de Minas Gerais (protocol 2/2015).
MSU‐induced gout
Crystals of monosodium urate (MSU) was prepared from uric acid (Sigma‐Aldrich, St. Louis, MO, USA) as previously described [3]. Mice under anaesthesia (80:15 mg/kg ketamine:xylazine; i.p. Syntec, São Paulo, Brazil) were injected into the tibiofemoral knee joint with 30μg of MSU crystals. Inflammatory parameters were evaluated at different time‐points as indicated in each figure legend. Mice were euthanized, and the knee cavity was washed with PBS/BSA 3% (2 × 5 μL) to collect the cells. The total number of leucocytes was determined using the Neubauer chamber after staining with Turk's solution. The differential counts were performed using standard morphologic criteria on a slide stained with May–Grünwald–Giemsa stain. Periarticular tissues were collected from the joints for evaluation of cytokines.
Assessment of CD300a expression
Mice were injected with 30 µg of MSU crystals into the tibiofemoral knee joint. Cells from the knee cavity were harvested and surface‐stained with Ly6G‐BV421 (BioLegend, San Diego, CA, USA, Clone 1A8) and anti‐CD300a‐PE (R&D Systems, Clone #172224). Blood samples from the same mice were collected to be used as a control group. Red cells were lysed using ACK solution (NH4Cl 150 mmol/L, KHCO3 10 mmol/L, Na2EDTA 0·1 mmol/L) and surface‐stained with Ly6G‐BV421 (BioLegend, San Diego, CA, USA) and anti‐CD300a‐PE (R&D Systems). Expression of CD300a receptor was assessed by flow cytometry on BD FACSCanto II (BD Bioscience, San Diego, CA, USA).
Cytokine measurement
Periarticular tissue was collected and homogenized in PBS containing antiproteases, as previously described [4]. The concentration of IL‐1β and CXCL1 was measured by ELISA in the supernatant of the homogenates and according to the instructions of the manufacturer (R&D Systems). Results are expressed in pg/mL.
Evaluation of hypernociception
The mechanical hypernociception was evaluated as previously described [4] using an electronic pressure meter (Insight Instruments, Ribeirao Preto, SP, Brazil). The dorsiflexion‐elicited withdrawal threshold was expressed in grams (g) and used to infer behavioural responses associated with experimental pain (hypernociception).
Histological analysis
Samples were processed as previously described [20]. Briefly, knee joints were collected, fixed in 10% formol and decalcified for 30 days in 14% EDTA. Tissues were included in paraffin, sectioned (5μm) and stained with H&E. Two sections of knee joints were examined and scored by a pathologist (C.M.Q‐J.) who was unaware of the experimental groups. The parameters evaluated were as follows: severity of synovial hyperplasia, intensity and extension of inflammatory infiltrate, vascular hyperaemia, presence of inflammatory cells in the synovial cavity and changes in tissue architecture. These criteria ranged from 0 to 8 points, and the sum was used to obtain a histological score.
Assessment of apoptosis
Apoptosis was assessed morphologically, as we reported previously [21]. Briefly, cells collected 12 hr after MSU crystal injection in WT and CD300a−/− mice were cytocentrifuged, fixed and stained with May–Grünwald–Giemsa stain and counted using oil immersion microscopy (X100 objective) to determine the proportion of cells with distinctive apoptotic morphology in a blinded manner. Apoptosis was also evaluated by flow cytometry. We injected MSU crystals into the peritoneal cavity, and 3 hr later, we performed a peritoneal lavage to recover recruited neutrophils. Neutrophils were separated through a double‐density gradient using Histopaque® 10,771 and 11,191 (Sigma‐Aldrich, St. Louis, MO, USA) and incubated in RPMI with 10% FBS, without stimuli, at 37º C to assess spontaneous apoptosis. Cells were collected and surface‐stained for 30 min with anti‐LY6G‐BV421 antibody (BioLegend, San Diego, CA, USA, Clone 1A8) and then labelled with annexin V APC and PI as an index of loss of nuclear membrane integrity (Annexin V APC Apoptosis Detection Kit; BD PharmingenTM; San Jose, CA, USA, Clone #550474). Apoptosis was assessed by flow cytometry on BD FACSCanto II (BD Bioscience, San Diego, CA, USA) using the frequency of LY6G+/annexin V+/PI− cells using the FlowJo software.
Efferocytosis assays
For the in vivo efferocytosis assays, joint wash was performed 24 hr after the MSU injection and cells were surface‐stained for 30 min with anti‐F4/80‐PECy7 antibody (BioLegend, San Diego, CA, USA, Clone BM8). Then, cells were fixed for 10 min, treated with 1× permeabilization wash (Cytofix/Cytoperm Kit; BD Biosciences) and intracellularly stained with anti‐Ly6G‐BV421 antibody (BioLegend, San Diego, CA, USA, Clone 1A8). Macrophage efferocytosis was assessed as a frequency of macrophages containing neutrophils (F4/80+ Ly6G+ cells) as previously described [22]. For the ex vivo efferocytosis assay, mice received an intraperitoneal injection of 0·1 mg of zymosan. To recover macrophages, cells were harvested from peritoneum 3 days after injection, isolated through double‐density gradient using Histopaque® 10,771 and 11,191 (Sigma‐Aldrich, St. Louis, MO, USA) and surface‐stained for 30 min with anti‐F4/80‐PECy7 antibody (BioLegend, San Diego, CA, USA, Clone BM8). To recover neutrophils, cells were harvested from peritoneum 3 hr after injection, and incubated at 37° and 5% CO2 with 10 μmol/L staurosporine (Sigma‐Aldrich, St. Louis, MO, USA) and 5 μmol/L CFSE (carboxyfluorescein diacetate succinimidyl ester; Life Technologies, Carlsbad, CA, USA) for 30 min. Neutrophil apoptosis was confirmed using morphology criteria in cytospin slides. Efferocytosis by adherent macrophages was assessed by flow cytometry on BD FACSCanto II (BD Bioscience, San Diego, CA, USA). The analyses were performed using the frequency of F480+/CFSE+ cells as previously described [23] using the FlowJo software. All flow cytometry experiments are in accordance with the journal guidelines [24].
Isolation of human peripheral blood neutrophils
A blood sample was collected from healthy volunteer donors (Ethics Committee on Human Research of Universidade Federal de Minas Gerais (COEP/UFMG protocol no. 0319.0.203.000‐11)) in tubes with EDTA‐containing Vacuette® system (3·2%), and neutrophils were isolated through double‐density gradient using Histopaque® 10 771 and 11 191 (Sigma‐Aldrich, St. Louis, MO, USA) as previously described [11]. Neutrophils were incubated at 37° and 5% CO2 with or without mouse anti‐human CD300a (Hybridoma #12, produced in‐house, Jerusalem) agonistic antibody for 5 and 15 min and for 8 hr, to perform Western blot analysis and flow cytometry analysis, respectively. Cells were washed with PBS and were either lysed to perform Western blot analysis or labelled with annexin V APC and PI as an index of loss of nuclear membrane integrity (Annexin V APC Apoptosis Detection Kit; BD PharmingenTM, San Jose, CA, USA, Clone #550474) to access apoptosis by flow cytometry.
Western blot analysis
Whole‐neutrophil extracts were prepared as described [25]. Proteins were extracted using lysis buffer containing antiproteases, and the amounts were quantified with the Bradford assay reagent from Bio‐Rad (Hercules, CA, USA). Extracts (40 μg) were separated by electrophoresis on a denaturing 12% polyacrylamide–SDS gel and electrotransferred to nitrocellulose membranes. Membranes were incubated with specific primary antibodies (anti‐cleaved caspase‐8; Cell Signaling Technology, Beverly, MA, USA, Clone #9748) and then incubated with appropriated HRP‐conjugated secondary antibody. Immunoreactive bands were visualized by using an ECL detection system, as described by the manufacturer (GE Healthcare, Piscataway, NJ, USA). For loading control, membranes were reprobed with anti‐GAPDH (Cell Signaling Technology, Beverly, MA, USA, Clone #3683).
Statistical analysis
All results are presented as the mean ± SEM. The analysis of the difference between two groups was performed by two‐tailed unpaired Student's t‐test. Normalized data were analysed by one‐way ANOVA, and differences between groups were assessed using the Newman–Keuls multiple comparison post hoc test. A p‐value <0·05 was considered significant. Calculations were performed using the Prism 7.0 software programme for Windows (GraphPad software, San Diego, CA, USA).
RESULTS
Injection of MSU crystals into the knee joint of mice leads to increased expression of CD300a on the recruited neutrophils
As we have previously reported, injection of 100 µg of MSU crystals into the knee joint causes articular inflammation with an influx of leucocytes after 12 hr, mainly neutrophils [4]. In this work, we used a suboptimal dose of MSU crystals (30 µg) to observe whether there could be enhancement of inflammatory cell recruitment. As seen in Figures 1 and 2, at this dose, we also observe articular inflammation and leucocyte recruitment 12h after the injection. We first investigated the expression of CD300a receptor on neutrophils recruited into the synovial cavity after MSU crystal injection. As blood neutrophils are quiescent and the presence of neutrophils is not observed in a non‐inflamed synovial cavity [26], we compared CD300a receptor expression among blood neutrophils and neutrophils present in the inflamed cavity. Using Ly6G as a neutrophil marker, CD300a receptor expression was measured in positive Ly6G cells by flow cytometry. Virtually, all neutrophils (96%–99%) expressed CD300a receptor in both blood and lavage samples (data not shown). Nevertheless, the intensity of CD300a expression was higher, in neutrophils obtained from the inflamed cavity in comparison with blood neutrophils, as measured by median fluorescence intensity (MFI) (Figure 1).
FIGURE 1.
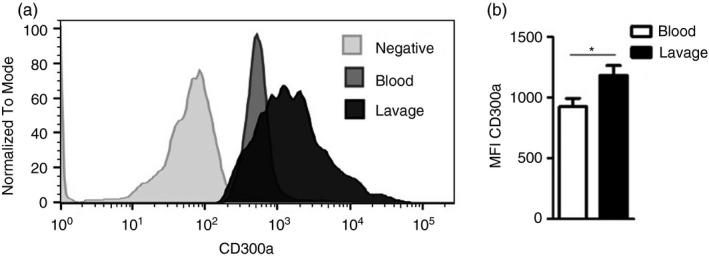
CD300a expression on neutrophils from blood and synovial cavity. BALB/c male mice were injected with 30 µg of MSU crystals into the tibiofemoral joint. Cells were harvested from the blood and articular cavity 12h after injection to measure CD300a expression on neutrophils by flow cytometry. Neutrophils were stained with anti‐Ly6G, and CD300a expression was evaluated on positive Ly6G cells (a). The median fluorescence intensity (MFI) of CD300a was measured on the neutrophils from the blood and synovial cavity (b). Bars show the mean ± SEM of 6 mice per group and are from one experiment representative of two independent experiments. Significance was calculated in relation to the control group (two‐tailed unpaired Student's t‐test). *p < 0·05
FIGURE 2.
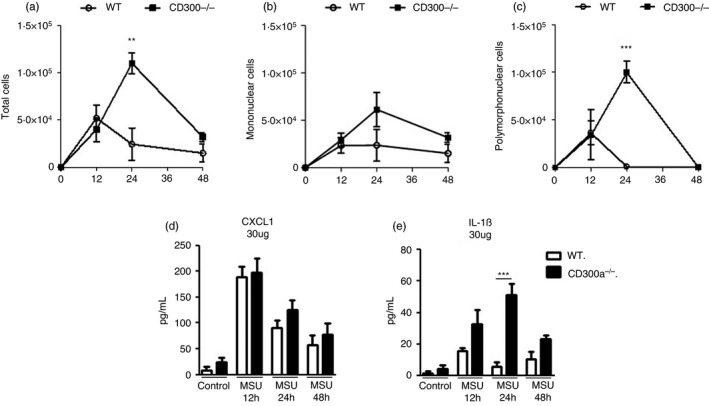
Time course of cell infiltrate and inflammatory mediators in acute gout inflammation. BALB/c and CD300a−/− male mice were injected with MSU crystals into the tibiofemoral joint. Cells from the articular cavity and periarticular tissue were harvested at 12, 24 and 48 hr after injection. From the articular cavity, total cells (a), mononuclear cells (b) and polymorphonuclear cells (c) were analysed. Polymorphonuclear and mononuclear cell populations were determined in cytospin preparation count. Periarticular tissue was processed, and the inflammatory chemokine CXCL1 (d) and the inflammatory cytokine IL‐1β (e) were measured. Bars show the mean ± SEM of 6 mice per group and are from one experiment representative of two independent experiments. Significance was calculated using ANOVA followed by the Newman–Keuls test. **p < 0·005, *** p < 0·001
Mice lacking CD300a presents delayed resolution of inflammation and increased IL‐1β production
After measuring the expression of the CD300a on neutrophils recruited into the synovial cavity 12 hr after injection with MSU, we analysed whether the absence of CD300a receptor could influence leucocyte recruitment in this model. We performed a time course of leucocyte infiltration in the knee cavity in wild‐type (WT) and CD300a knockout (CD300a−/−) mice at 12, 24 and 48 hr after MSU injection. There was similar recruitment of leucocytes, mostly neutrophils, at 12 hr after injection of MSU crystals in both WT and CD300a−/− mice. A crucial difference was observed at the 24‐hr time‐point, the time when the spontaneous resolution of inflammation occurs in this model [27]. Whereas the number of neutrophils in the knee joint of WT mice had virtually returned to background levels at 24 hr, neutrophils were even in greater number at this time in the knee joint of CD300a−/− mice injected with MSU crystals (Figure 2). Eventually, CD300a−/− mice also resolved inflammation as there were no neutrophils in the knee joint at 48 hr after injection of MSU crystals in these mice. At this time‐point, only mononuclear cells were present (Figure 2b).
We evaluated the production of the chemokine CXCL1 and the cytokine IL‐1β in periarticular tissue. Both cytokines are crucial mediators to drive leucocyte influx into the joint [4]. While CXCL1 production was similar in all time‐points, IL‐1β production by CD300a−/− mice was greater at the 24‐hr time‐point in comparison with their WT counterparts (Figure 2d, e). These data suggest that the absence of CD300a receptor worsens MSU crystal‐induced inflammation.
Lack of CD300a leads to increased tissue damage but no change in hypernociception
Histopathological analysis of the knees from WT and CD300a−/− mice was performed 24 hr after MSU injection. The analysis showed that the inflammatory infiltrate, synovial hyperplasia and hyperaemia of vessels were greater in CD300a−/− mice as compared to WT mice, as shown by the total histopathologic score (Figure 3a, b). Hypernociception was analysed to investigate the degree of articular dysfunction after injection of MSU crystals. Injection of 30 µg of MSU crystals was sufficient to generate almost maximal hypernociception in the system, and there was no significant increase in this parameter in CD300a−/− mice (Figure 3c). These data suggest that the absence of CD300a receptor increases tissue damage induced by MSU crystals but not hypernociception.
FIGURE 3.
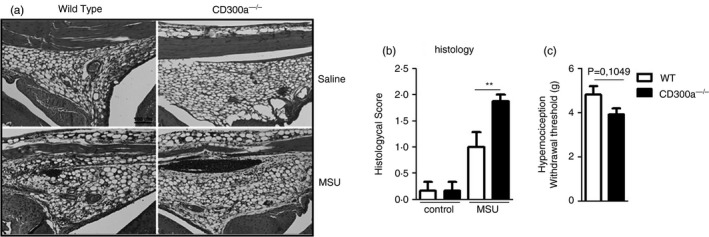
Histopathological analysis and articular dysfunction. BALB/c and CD300a−/− male mice were injected with MSU crystals into the tibiofemoral joint. Histopathologic analysis (magnification of 100×) was observed 24 hr after MSU crystal injection by criteria previously described (a) (scale bar: 100 µm). Semiquantitative analysis of histological findings is also shown (b). To evaluate articular dysfunction, hypernociception was evaluated using an electronic pressure meter test at 24 hr after injection. Bars show the mean ± SEM of 6 mice per group and are from one experiment representative of two independent experiments. Significance was calculated using the ANOVA followed by the Newman–Keuls test and two‐tailed unpaired Student's t‐test in relation to the control group (the exact p‐value is shown in the figure). **p < 0·005
Apoptosis is impaired in CD300a−/− neutrophils
During routine counting of migrated cells, we observed a larger amount of neutrophils with apoptotic morphology in WT when compared to the CD300a−/− ones. This observation sheds light on the possible mechanism that leads to the worse inflammation of CD300a−/− mice. To analyse whether WT neutrophils were becoming apoptotic before CD300a−/− neutrophils, we counted the number of apoptotic neutrophils in the synovial lavage obtained 12 hr after injection with MSU crystals. As seen in Figure 4, we found an increased percentage of apoptotic neutrophils in WT samples as compared to CD300a−/− samples (Figure 4).
FIGURE 4.
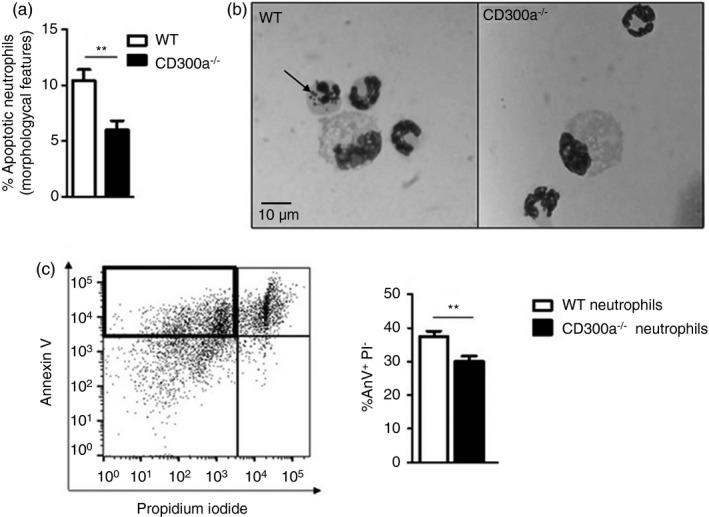
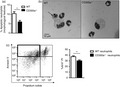
Difference in apoptosis among wild‐type and CD300a−/− neutrophils. BALB/c and CD300a−/− male mice were injected with MSU crystals into the tibiofemoral joint. Cells from the articular cavity were harvested 24 hr after injection, and cells with distinctive apoptotic morphology were evaluated on cytospin and expressed as per cent of neutrophil with apoptotic morphology (a). Representative figures (magnification of 1000×) of viable and apoptotic neutrophil (arrow) (b). BALB/c and CD300a−/− male mice were injected with MSU crystals into the peritoneal cavity. Neutrophils were recovered and isolated 3 hr after peritoneal injection and incubated for 24 hr at 37°. After incubation, neutrophils were stained with annexin V and propidium iodide to assess early apoptosis through flow cytometry (c). The dot plot is an example of general analysis of early apoptosis. Bars show the mean ± SEM of 7 mice per group and are from one experiment representative of two independent experiments. Significance was calculated in relation to the control group (two‐tailed unpaired Student's t‐test). **p < 0·005
To confirm these data, we injected MSU crystals into the peritoneal cavity, and 3 h later, we performed a peritoneal lavage to recover recruited neutrophils. We used the peritoneal cavity as it is not possible to obtain neutrophils from the knee joint to conduct subsequent ex vivo experiments. Neutrophils were separated by Histopaque® gradient and incubated in RPMI with 10% FBS, without stimuli, at 37° to access spontaneous apoptosis. After 24 hr, neutrophils were stained with the neutrophil marker Ly6G, annexin V (AnxV) and propidium iodide (PI). Ly6G‐positive population was gated, and spontaneous early apoptosis was defined as AnxV+/PI− events, as previously reported [28, 29]. The gating strategy is demonstrated in Supplementary Figure S1. Corroborating the data from synovial lavage of mice, there were more apoptotic WT neutrophils than apoptotic CD300a−/− neutrophils at 24 hr after ex vivo culture (Figure 4c).
Efferocytosis is hindered when cells lack CD300a
One of the key events of the resolution of inflammation is the clearance of apoptotic neutrophils, a process called efferocytosis [17]. Having observed a decrease in number of apoptotic neutrophils in CD300a−/− mice, we investigated whether this phenomenon was associated with an altered efferocytic process. We evaluated macrophage efferocytosis in cells harvested from the joint cavity of WT and CD300a−/− mice at 24‐hr time‐point. We observed a larger increase in the number of efferocytic events in the lavage of WT mice when compared to lavage from CD300a−/− mice (Figure 5a). To confirm these data, we performed an ex vivo experiment, where neutrophils from both WT and CD300a−/− mice were incubated with WT macrophages. Neutrophils were stained with CFSE and incubated with staurosporine for 30 min to induce apoptosis as previously described [30]. Both WT and CD300a−/− neutrophils incubated with staurosporine became apoptotic in similar percentages (Supplementary Figure S6). Thereafter, apoptotic neutrophils were incubated with macrophages from WT mice for 1 hr and efferocytosis was evaluated by flow cytometry. We observed that CD300a−/− neutrophils were less phagocytosed than WT neutrophils (Figure 5b). The gating strategy of these experiments is shown in Supplementary Figures S2 and S3. Taken together, these results suggest that the absence of CD300a receptor decreased neutrophil apoptosis and efferocytosis leading to delayed resolution of inflammation.
FIGURE 5.
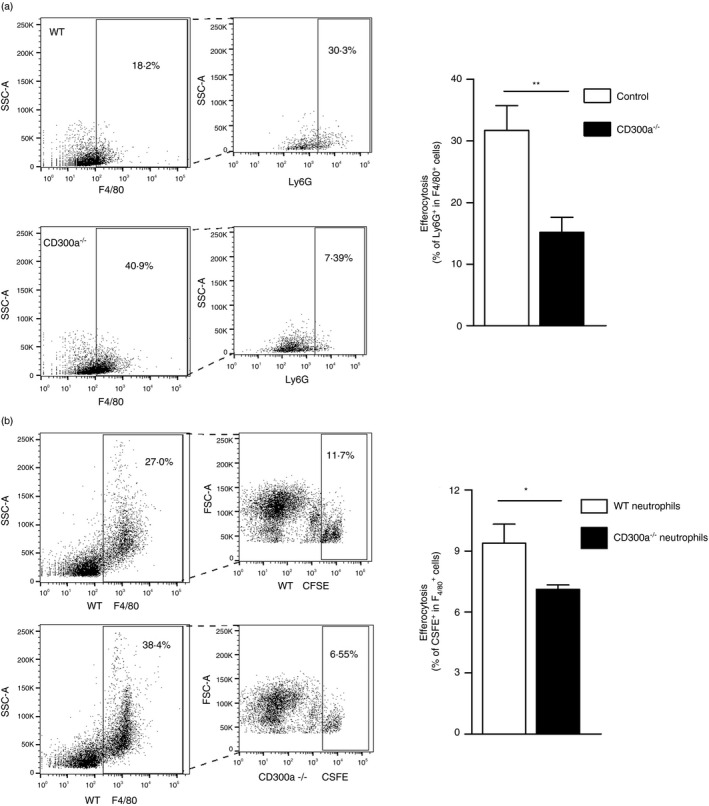
Effects of the deletion of CD300a on efferocytosis. The in vivo efferocytosis analysis was performed in BALB/c (WT) and CD300a−/− male mice injected with MSU crystals into the tibiofemoral joint. Cells from the articular cavity were harvested 24 hr after injection, stained with surface marker F4/80, permeabilized and intracellularly stained with Ly6G. Efferocytosis was assessed by flow cytometry analysing F4/80+Ly6G+ (a). The ex vivo efferocytosis analysis was performed in BALB/c (WT) and CD300a−/− male mice injected with zymosan into the peritoneal cavity. Cells were recovered, and neutrophils were separated by Histopaque gradient. Apoptotic neutrophils from WT and CD300a−/− labelled with fluorescent CFSE were incubated with WT macrophages in a proportion of 3 neutrophils per macrophage. Efferocytosis was assessed by flow cytometry analysing F4/80+CFSE+ (b). Bars show the means ± SEM of 5 mice per group and are from one experiment representative of two independent experiments. Significance was calculated in relation to the control group (two‐tailed unpaired Student's t‐test). *p < 0·05 and **p < 0·005
CD300a activation leads to neutrophil apoptosis and caspase‐8 cleavage
The results so far suggest that a decrease in apoptosis of CD300a−/− neutrophils contribute to the delayed resolution of inflammation in these animals. To evaluate whether neutrophils undergo apoptosis upon direct CD300a ligation, we isolated neutrophils from healthy human donors and incubated them with or without a CD300a agonistic antibody (α‐CD300a). In neutrophils incubated with the α‐CD300a for 8 h, there was a greater increase in the AnxV+/PI− population (early apoptosis) than in control neutrophils, as shown by flow cytometry (Figure 6a). The gating strategy for these experiments is shown in Supplementary Figure S4. Next, we investigated whether caspase‐8 was activated by activation of CD300a. Caspase‐8 activation is a central mechanism in the control of neutrophil apoptosis during the resolution of inflammation [31]. Blood from healthy human donors was collected, and neutrophils were isolated and incubated with or without α‐CD300a for 5 and 15 min. The cross‐linking of CD300a receptor increased caspase‐8 activation, seen as cleaved caspase‐8 (Figure 6b, c), suggesting that the CD300a receptor activates this pathway to induce apoptosis in neutrophils. The entire blot is shown in Supplementary Figure S5.
FIGURE 6.
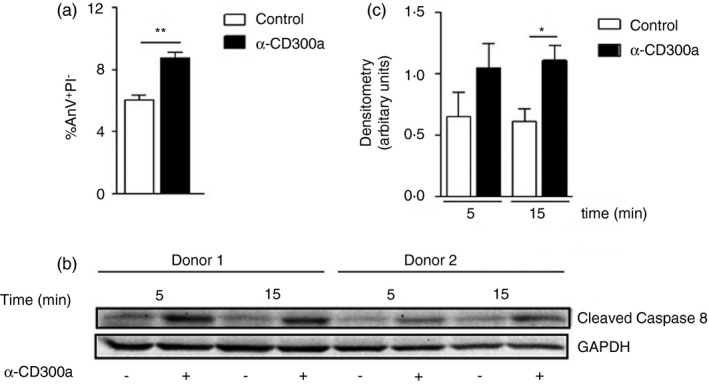
Apoptosis and cleavage of caspase‐8 on human blood neutrophils stimulated with anti‐CD300a agonistic antibody. Neutrophils were purified from the blood of human donors, and 1 × 105 cells were incubated with or without human agonistic CD300a antibody (α‐CD300a) to assess early neutrophil apoptosis (a). Neutrophils were purified from the blood of human donors, and 2 × 106 cells were incubated with or without α‐CD300a. Total cell lysates were separated by SDS‐PAGE and subjected to Western blotting for cleaved caspase‐8 detection after 5 and 15 min of incubation. The image represents one of two independent experiments (b), and the densimetry of two independent experiments is shown (c). Significance was calculated using ANOVA followed by the Newman–Keuls test and two‐tailed unpaired Student's t‐test in relation to the control group. *p < 0·05 and **p < 0·005
DISCUSSION
In the present work, we investigated how the inhibitory receptor CD300a participates in the control of articular inflammation triggered by intra‐articular injection of MSU crystals in mice. We found that CD300a interferes with the apoptosis of neutrophils, which, in turn, influences the resolution of inflammation. Our major findings can be summarized as follows: (i) higher expression of CD300a was detected in neutrophils recruited into the cavity injected with MSU crystals; (ii) mice lacking CD300a had delayed resolution of inflammation associated with greater IL‐1β production and greater histopathological score, when compared to WT mice; (iii) neutrophils from CD300a−/− mice had impaired apoptosis, and this was associated with less efferocytosis of these cells; (iv) mechanistically, CD300a activation induced apoptosis through activation of pro‐apoptotic caspase‐8, which can elucidate the mechanism by which CD300a acts on resolution of inflammation.
The inflammatory response is of paramount importance to control infections and to repair tissues, but the response needs to be strictly regulated in order to prevent a pathological response, which could lead to tissue damage [32]. A successful inflammatory response ends with the elimination of the stimuli and the gradual return to homeostasis, a process called resolution of inflammation [33]. Current studies on resolution of inflammation aim to find ways to restore tissue homeostasis by using pro‐resolving mediators and, more recently, by studying mechanisms involving inhibitory receptors [19, 34, 35, 36]. CD300a is an increasingly studied inhibitory receptor that is shown to have an anti‐inflammatory role in the context of several models of inflammatory disease, including antigen‐induced arthritis [11], atopic dermatitis [19] and allergic models [18, 37]. CD300a has been demonstrated to be important to limit the function of eosinophils, basophils and mast cells [19, 38], but much less is known about the importance of CD300a on neutrophils, one of the most important leucocytes in the process of inflammation and resolution of inflammation [17, 39]. Here, we demonstrate, for the first time, how the immunoreceptor CD300a exerts an important function on the resolution of neutrophilic inflammation induced by MSU crystals, mainly by acting on neutrophils.
It is known that inflammatory stimuli enhance CD300a expression both in vitro and in vivo [11, 18, 40]. When MSU was injected into the synovial cavity of mice, we observed higher CD300a expression on neutrophils recruited into the knee than on neutrophils obtained from the blood, where they are considered quiescent [26]. This result is in agreement with other studies that showed increased CD300a expression after cell activation [40] and migration [11, 41], suggesting that CD300a has an important role in activated cells. Although there is evidence of CD300a upregulation in monocytes after transmigration [41], in this study we focused on the relevance of CD300a for neutrophils.
Using a low dose of MSU crystals (30 µg), we observed that in WT mice leucocyte, mostly neutrophil, recruitment peaks at 12 hr after the injection and is almost completely resolved after 24 hr. In contrast, neutrophils continued to be recruited, reaching their peak 24 hr after injection and just resolving at the 48‐hr time‐point. Similar data were observed in the work of Karra et al. where CD300a−/− mice showed delayed resolution of eosinophilic inflammation in a model of murine allergic peritonitis [18]. Gout is dependent on inflammasome activation, with subsequent production of IL‐1β [2, 4, 42]. Levels of IL‐1β in the periarticular tissue of CD300a−/− mice were higher when compared to WT mice 24 hr after MSU injection. At this same time‐point, there was a significant difference in leucocyte infiltration between both groups and neutrophils were the main type of leucocyte. This is consistent with our studies showing that in addition to macrophages, neutrophils contribute to IL‐1β production after injection of MSU crystals [4, 43]. In our previous work, in an antigen‐induced arthritis model, we found greater levels of the chemokine CXCL1, the main neutrophil chemo‐attractant, in knockout than in WT neutrophils [11]. Surprisingly, here we observed no difference in CXCL1 levels. This difference between the models can be due to the different stimulus and the low dose of MSU used in this work. This is consistent with the similar number of neutrophils at 12 hr. It appears therefore that greater neutrophil accumulation in CD300a−/− mice is not to secondary to greater recruitment, but it is rather due to their persistence stay in the joint cavity.
Different groups have reported a more intense inflammation in mice lacking the CD300a receptor [11, 18, 19, 44]. Here, histopathological analysis made at 24‐h time‐point evidenced the difference in inflammation level between WT and CD300a−/− mice. Inflammatory infiltration, moderate synovial hyperplasia and hyperaemia of vessels were higher in CD300a−/− mice. IL‐1β production is associated with increased mechanical hypernociception in mice [4, 45]. These increased parameters, along with a larger production of IL‐1β by CD300a−/− mice, lead us to investigate whether difference in hypernociception could be found. Although a slight tendency of CD300a−/− mice to present increased hypernociception was observed when compared to WT mice, no statistical difference was observed between the groups.
Inhibitory receptors, such as Siglec‐8 and Siglec‐9, are frequently associated with increased apoptosis of myeloid cells [46, 47]. In vitro antibody cross‐linking of Siglec‐9 results in increased apoptosis of resting neutrophils [48]. CD300a was shown to negatively regulate IL‐5‐induced human peripheral blood eosinophil survival, accelerating the apoptosis process [49]. Indeed, differential counting identified a substantial difference in the number of apoptotic neutrophils harvested from the synovial lavage of WT and CD300a−/− mice. WT mice showed a higher percentage of apoptotic neutrophils in the synovial lavage when compared to CD300a−/− mice. In vitro experiment of spontaneous apoptosis of neutrophils confirmed these data. An increased percentage of WT apoptotic neutrophils was observed when compared to CD300a−/− apoptotic neutrophils. Here, we considered early apoptosis when AnxV was positive and PI was negative, hence avoiding the inclusion of necrotic cells. Our finding is novel in that to the best of our knowledge, it has not been previously reported that CD300a is a receptor involved in neutrophil apoptosis process. Here, we showed for the first time that CD300a−/− neutrophils have a delay in the apoptosis process when compared to WT neutrophils.
Gouty arthritis is characterized by spontaneous resolution in both humans and mice [25, 50]. The resolution of inflammation is an active process that requires removal of leucocytes from inflamed sites. Neutrophils emigrated to the inflamed site undergo apoptosis with subsequent recognizing and phagocytosis by macrophages, in a process called efferocytosis [51, 52]. Due to the crucial importance of the clearance of apoptotic cells for a successful resolution of inflammation, we checked whether the efferocytosis process was altered in CD300a−/− mice. We observed less efferocytic events in CD300a−/− mice compared with WT mice, suggesting CD300a−/− mice have a delay in the resolution of articular inflammation by reduction in neutrophil apoptosis and its subsequent efferocytosis.
Although we found that CD300a receptor has a role in both the two important events for resolution of inflammation, apoptosis and efferocytosis, Nakahashi‐Oda et al. showed that compared with WT macrophages, macrophages lacking CD300a do not present differences in phagocytosis apoptotic cells [53]. We also have recently found that CD300a−/− macrophages present increased activation features, such as higher release of cytokines, and reduced possibility to switch towards a pro‐resolution M2 phenotype (PG, Puzzovio and F, Levi‐Schaffer, unpublished data). This evidence, along with our data, suggests that CD300a receptor may have a more significant role in neutrophils. We are not excluding the role of other cell types in the resolution of inflammation. However, as neutrophil influx into synovial fluid and cavity is the hallmark of gout and contributes to the pathogenesis of the disease, studying the importance of the CD300a in neutrophils could contribute to understand how CD300a could be a potential target to modulate resolution of neutrophilic inflammation.
Physiologically, phosphatidylserine (PS) and phosphatidylethanolamine (PE) bind and activate CD300a, which become able to recruit and activate phosphatases, including SHP‐1 [54, 55, 56, 57]. There are evidences that activation of CD300a with agonistic antibody also leads to SHP‐1 activation [12, 49, 58], playing a similar role of PS and PE binding. SHP‐1 phosphatase was described to have a crucial role in neutrophil apoptosis, mainly by activating caspase‐8 [59, 60, 61, 62]. Apoptosis can be initiated by two alternative pathways: either through death receptors on the cell surface (extrinsic pathway) or through mitochondria (intrinsic pathway). In both pathways, induction of apoptosis leads to activation of an initiator caspase: caspase‐8 and possibly caspase‐10 for the extrinsic pathway; and caspase‐9, which is activated at the apoptosome, for the intrinsic pathway. The initiator caspases then activate executioner caspases [63]. Caspase‐8 is one of the main upstream caspase effector of death receptor signalling involved in the extrinsic pathway of apoptosis [64]. A previous study showed that the CD300a agonistic antibody used in this work led to SHP‐1 activation in eosinophils [65]. Here, we show that CD300a activation, using an agonist antibody, anticipates neutrophil apoptosis, observed by increased expression of AnxV. This antibody inhibited tryptase activity in a dose‐dependent manner in mast cells stimulated with IgE (not shown), suggesting its efficient action on activation of CD300a. Moreover, activation of CD300a leads to caspase‐8 cleavage in human neutrophils, suggesting that activation of CD300a may induce apoptosis via the extrinsic pathway.
In conclusion, the present study shows the importance of the CD300a in the resolution of MSU‐induced articular inflammation through modulation of neutrophil recruitment, and IL‐1β production and participation in the process of neutrophil apoptosis and efferocytosis. Mechanistically, CD300a activation induces apoptosis in neutrophils via activation of caspase‐8 cleavage. Our findings provide important insights into the mechanisms of resolution of inflammation, making CD300a a potential target to accelerate this process and help to control diseases.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
B.V.S.V., I.G. and M.M.T., designed research and wrote the paper. B.V.S.V. and I.G. performed experiments and analysed data. C.M.Q‐J. performed histology analysis. F.L‐S. provided suggestions and comments on CD300a and corrected the paper draft. All authors have read and agreed to the published version of the manuscript.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
ACKNOWLEDGEMENTS
We would like to thank Ilma Marçal, Rosemeire Oliveira, Linelinda Magalhães and Frankcineia Assis for technical assistance (Brazil), Laila Karra for backcrossing the CD300a‐/‐ mice and Pier Giorgio Puzzovio for comments on the final draft of the manuscript (Israel).
Valiate BVS, Queiroz‐Junior CM, Levi‐Schaffer F, Galvão I, Teixeira MM. CD300a contributes to the resolution of articular inflammation triggered by MSU crystals by controlling neutrophil apoptosis. Immunology. 2021;164:305–317. 10.1111/imm.13371
Galvão and Teixeira contributed equally.
Funding information
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil), Pró‐Reitoria de Pesquisa da Universidade Federal de Minas Gerais‐PRPq, Brazil, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Rosetrees Trust UK and Israel Science Foundation (to F.L‐S).
REFERENCES
- 1.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–62. 10.1038/nrrheum.2015.91 [DOI] [PubMed] [Google Scholar]
- 2.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Shi Y, Hearn A, et al. MyD88‐dependent IL‐1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–71. 10.1172/JCI28075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral FA, Costa VV, Tavares LD, et al. NLRP3 inflammasome‐mediated neutrophil recruitment and hypernociception depend on leukotriene B4 in a murine model of gout. Arthritis Rheum. 2012;64:474–84. 10.1002/art.33355 [DOI] [PubMed] [Google Scholar]
- 5.Popa‐Nita O, Naccache PH. Crystal‐induced neutrophil activation. Immunol Cell Biol. 2010;88:32–40. 10.1038/icb.2009.98 [DOI] [PubMed] [Google Scholar]
- 6.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84‐9. Accessed March 18, 2017. http://www.ncbi.nlm.nih.gov/pubmed/11021804 [DOI] [PubMed] [Google Scholar]
- 7.Clark GJ, Ju X, Tate C, Hart DNJ. The CD300 family of molecules are evolutionarily significant regulators of leukocyte functions. Trends Immunol. 2009;30:209–17. 10.1016/j.it.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 8.Borrego F. The CD300 molecules: an emerging family of regulators of the immune system. Blood. 2013;121:1951–60. 10.1182/blood-2012-09-435057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachelet I, Munitz A, Levi‐Schaffer F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol. 2006;117:1314–20. 10.1016/j.jaci.2006.04.031 [DOI] [PubMed] [Google Scholar]
- 10.Silva R, Moir S, Kardava L, et al. CD300a is expressed on human B cells, modulates BCR‐mediated signaling, and its expression is down‐regulated in HIV infection. Blood. 2011;117:5870–80. 10.1182/blood-2010-09-310318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valiate BVS, Alvarez RU, Karra L, et al. The immunoreceptor CD300a controls the intensity of inflammation and dysfunction in a model of Ag‐induced arthritis in mice. J Leukoc Biol. 2019;106:957–66. 10.1002/JLB.3A1018-389R [DOI] [PubMed] [Google Scholar]
- 12.Cantoni C, Bottino C, Augugliaro R, et al. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol. 1999;29:3148–59. 10.1002/(SICI)1521-4141(199910)29:10%3c3148:AID-IMMU3148%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 13.Nielsen R, Bustamante C, Clark AG, et al. A scan for positively selected genes in the genomes of humans and chimpanzees. Tyler‐Smith C, ed. PLoS Biol. 2005;3:e170. 10.1371/journal.pbio.0030170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bustamante CD, Fledel‐Alon A, Williamson S, et al. Natural selection on protein‐coding genes in the human genome. Nature. 2005;437:1153–7. 10.1038/nature04240 [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–7. 10.1038/ni1276 [DOI] [PubMed] [Google Scholar]
- 16.Headland SE, Norling LV. The resolution of inflammation: Principles and challenges. Semin Immunol. 2015;27:149–60. 10.1016/j.smim.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: What controls its onset? Front Immunol. 2016;7:160. 10.3389/fimmu.2016.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karra L, Singh Gangwar R, Shamri R, et al. Leukocyte CD300a contributes to the resolution of murine allergic inflammation. J Immunol. Published online. 2018;201:2998–3005. 10.4049/jimmunol.1801000 [DOI] [PubMed] [Google Scholar]
- 19.Karra L, Gangwar RS, Puzzovio PG, et al. CD300a expression is modulated in atopic dermatitis and could influence the inflammatory response. Allergy. 2019;74:1377–80. Published online February 19, 2019. 10.1111/all.13724 [DOI] [PubMed] [Google Scholar]
- 20.Queiroz‐Junior CM, Madeira MFM, Coelho FM, et al. Experimental arthritis triggers periodontal disease in mice: involvement of TNF‐ and the oral microbiota. J Immunol. 2011;187:3821–30. 10.4049/jimmunol.1101195 [DOI] [PubMed] [Google Scholar]
- 21.Vago JP, Nogueira CRC, Tavares LP, et al. Annexin A1 modulates natural and glucocorticoid‐induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012;92:249–58. 10.1189/jlb.0112008 [DOI] [PubMed] [Google Scholar]
- 22.Dalli J, Consalvo AP, Ray V, et al. Proresolving and tissue‐protective actions of annexin A1–based cleavage‐resistant peptides are mediated by formyl peptide receptor 2/lipoxin A 4 receptor. J Immunol. 2013;190:6478–87. 10.4049/jimmunol.1203000 [DOI] [PubMed] [Google Scholar]
- 23.Negreiros‐Lima GL, Lima KM, Moreira IZ, et al. Cyclic AMP regulates key features of macrophages via PKA: recruitment, reprogramming and efferocytosis. Cells. 2020;9:128. 10.3390/cells9010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cossarizza A, Chang H, Radbruch A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019;49:1457–973. 10.1002/eji.201970107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvão I, Vago JP, Barroso LC, et al. Annexin A1 promotes timely resolution of inflammation in murine gout. Eur J Immunol. 2017;47:585–96. 10.1002/eji.201646551 [DOI] [PubMed] [Google Scholar]
- 26.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol Mech Dis. 2014;9:181–218. 10.1146/annurev-pathol-020712-164023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvão I, Dias ACF, Tavares LD, et al. Macrophage migration inhibitory factor drives neutrophil accumulation by facilitating IL‐1β production in a murine model of acute gout. J Leukoc Biol. 2016;99:1035–43. 10.1189/jlb.3ma0915-418r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, Hong YS, Choi SH, Yoon YH, Moon SW, Lee SW. Effect of hypertonic saline on apoptosis of polymorphonuclear cells. J Surg Res. 2012;178:401–8. 10.1016/j.jss.2012.01.055 [DOI] [PubMed] [Google Scholar]
- 29.Majewska E, Sulowska Z, Baj Z. Spontaneous apoptosis of neutrophils in whole blood and its relation to apoptosis gene proteins. Scand J Immunol. 2000;52:496–501. 10.1046/j.1365-3083.2000.00802.x [DOI] [PubMed] [Google Scholar]
- 30.Belmokhtar CA, Hillion J, Ségal‐Bendirdjian E. Staurosporine induces apoptosis through both caspase‐dependent and caspase‐independent mechanisms. Oncogene. 2001;20:3354–62. 10.1038/sj.onc.1204436 [DOI] [PubMed] [Google Scholar]
- 31.Conus S, Perozzo R, Reinheckel T, et al. Caspase‐8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205:685–98. 10.1084/jem.20072152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. 10.1038/nature01320 [DOI] [PubMed] [Google Scholar]
- 33.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–67. 10.1038/nrd.2016.39 [DOI] [PubMed] [Google Scholar]
- 34.Ritzel RM, Al Mamun A, Crapser J, et al. CD200‐CD200R1 inhibitory signaling prevents spontaneous bacterial infection and promotes resolution of neuroinflammation and recovery after stroke. J Neuroinflammation. 2019;16: 10.1186/s12974-019-1426-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. 10.1038/nri2470 [DOI] [PubMed] [Google Scholar]
- 36.Stewart AG. Mediators and receptors in the resolution of inflammation: Drug targeting opportunities. Br J Pharmacol. 2009;158:933–5. 10.1111/j.1476-5381.2009.00484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miki H, Nakahashi‐Oda C, Sumida T, Shibuya A. Involvement of CD300a phosphatidylserine immunoreceptor in aluminum salt adjuvant‐induced Th2 responses. J Immunol. 2015;194:5069–76. 10.4049/jimmunol.1402915 [DOI] [PubMed] [Google Scholar]
- 38.Sabato V, Verweij MM, Bridts CH, et al. CD300a is expressed on human basophils and seems to inhibit IgE/FcεRI‐dependent anaphylactic degranulation. Cytom Part B Clin Cytom. 2012;82B:132–8. 10.1002/cyto.b.21003 [DOI] [PubMed] [Google Scholar]
- 39.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–45. 10.1016/j.smim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 40.Alvarez Y, Tang X, Coligan JE, Borrego F. The CD300a (IRp60) inhibitory receptor is rapidly up‐regulated on human neutrophils in response to inflammatory stimuli and modulates CD32a (FcγRIIa) mediated signaling. Mol Immunol. 2008;45:253–8. 10.1016/j.molimm.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghavampour S, Lange C, Bottino C, Gerke V. Transcriptional profiling of human monocytes identifies the inhibitory receptor CD300a as regulator of transendothelial migration. Kuwana M, editor. PLoS One. 2013;8:e73981. 10.1371/journal.pone.0073981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mian WU, Zhang M, Ma Y, et al. Chaetocin attenuates gout in mice through inhibiting HIF‐1α and NLRP3 inflammasome‐dependent IL‐1β secretion in macrophages. Arch Biochem Biophys. 2019;670:94–103. 10.1016/j.abb.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 43.Schett G, Dayer JM, Manger B. Interleukin‐1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12:14–24. 10.1038/nrrheum.2016.166 [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Tahara‐Hanaoka S, Nabekura T, et al. PPARβ/δ activation of CD300a controls intestinal immunity. Sci Rep. 2014;4:5412. 10.1038/srep05412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunha TM, Verri WA, Fukada SY, et al. TNF‐α and IL‐1β mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor. Eur J Pharmacol. 2007;573(1–3):221–9. 10.1016/j.ejphar.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 46.Gunten S, Simon H. Sialic acid binding immunoglobulin‐like lectins may regulate innate immune responses by modulating the life span of granulocytes. FASEB J. 2006;20:601–5. 10.1096/fj.05-5401hyp [DOI] [PubMed] [Google Scholar]
- 47.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec‐8: A selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–20. 10.1182/blood-2002-10-3058 [DOI] [PubMed] [Google Scholar]
- 48.Von Gunten S, Yousefi S, Seitz M, et al. Siglec‐9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106:1423–31. 10.1182/blood-2004-10-4112 [DOI] [PubMed] [Google Scholar]
- 49.Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi‐Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL‐5, GM‐CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107:1996–2003. 10.1182/blood-2005-07-2926 [DOI] [PubMed] [Google Scholar]
- 50.Steiger S, Harper JL. Mechanisms of spontaneous resolution of acute gouty inflammation. Curr Rheumatol Rep. 2014;16(1):392. 10.1007/s11926-013-0392-5 [DOI] [PubMed] [Google Scholar]
- 51.Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: Mechanisms and opportunity for drug development. Pharmacol Ther. 2013;139:189–212. 10.1016/j.pharmthera.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 52.El Kebir D, Filep J. targeting neutrophil apoptosis for enhancing the resolution of inflammation. Cells. 2013;2:330–48. 10.3390/cells2020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakahashi‐Oda C, Tahara‐Hanaoka S, Shoji M, et al. Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor. J Exp Med. 2012;209:1493–503. 10.1084/jem.20120096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachelet I, Munitz A, Moretta A, Moretta L, Levi‐Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989–95. Accessed March 13, 2017. http://www.ncbi.nlm.nih.gov/pubmed/16339535 [DOI] [PubMed] [Google Scholar]
- 55.DeBell KE, Simhadri VR, Mariano JL, Borrego F. Functional requirements for inhibitory signal transmission by the immunomodulatory receptor CD300a. BMC Immunol. 2012;13:23. 10.1186/1471-2172-13-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simhadri VR, Andersen JF, Calvo E, Choi S‐C, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119:2799–809. 10.1182/blood-2011-08-372425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakahashi‐Oda C, Tahara‐Hanaoka S, Honda S, Shibuya K, Shibuya A. Identification of phosphatidylserine as a ligand for the CD300a immunoreceptor. Biochem Biophys Res Commun. 2012;417:646–50. 10.1016/j.bbrc.2011.12.025 [DOI] [PubMed] [Google Scholar]
- 58.Kim EJ, Lee SM, Suk K, Lee WH. CD300a and CD300f differentially regulate the MyD88 and TRIF‐mediated TLR signalling pathways through activation of SHP‐1 and/or SHP‐2 in human monocytic cell lines. Immunology. 2012;135:226–35. 10.1111/j.1365-2567.2011.03528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Death receptors bind SHP‐1 and block cytokine‐induced anti‐apoptotic signaling in neutrophils. Nat Med. 2002;8:61–7. 10.1038/nm0102-61 [DOI] [PubMed] [Google Scholar]
- 60.Yousefi S, Simon HU. SHP‐1: A regulator of neutrophil apoptosis. Semin Immunol. 2003;15:195–9. 10.1016/S1044-5323(03)00033-2 [DOI] [PubMed] [Google Scholar]
- 61.Song HJ, Parodo J, Kapus A, Rotstein OD, Marshall JC. Dynamic regulation of neutrophil survival through tyrosine phosphorylation or dephosphorylation of caspase‐8. J Biol Chem. 2008;283:5402–13. 10.1074/jbc.M706462200 [DOI] [PubMed] [Google Scholar]
- 62.Liu D, Martino G, Thangaraju M, et al. Caspase‐8‐mediated intracellular acidification precedes mitochondrial dysfunction in somatostatin‐induced apoptosis. J Biol Chem. 2000;275:9244–50. 10.1074/jbc.275.13.9244 [DOI] [PubMed] [Google Scholar]
- 63.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruidering M, Evan G. Caspase‐8 in apoptosis: the beginning of “The End”? IUBMB Life. 2000;50:85–90. 10.1080/713803693 [DOI] [PubMed] [Google Scholar]
- 65.Legrand F, Landolina N, Zaffran I, et al. Siglec‐7 on peripheral blood eosinophils: Surface expression and function. Allergy Eur J Allergy Clin Immunol. 2019;74:1257–65. 10.1111/all.13730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6


