Abstract
Background
A new coronavirus (SARS-CoV-2) abruptly emerged in Wuhan, China, in 2019 and rapidly spread globally to cause the COVID-19 pandemic.
Aim
To examine the anti-SARS-CoV-2 activity of the potent disinfectant Cleverin, the major disinfecting component of which is chlorine dioxide (ClO2); and to compare the results with that of sodium hypochlorite in the presence or absence of 0.5% or 1.0% foetal bovine serum (FBS).
Methods
Concentrated SARS-CoV-2 viruses were treated with various concentrations of ClO2 and sodium hypochlorite and 50% tissue culture infective dose was calcurated to evaluate the antiviral activity of each chemical.
Findings
When SARS-CoV-2 viruses were treated with 0.8 ppm ClO2 or sodium hypochlorite, viral titre was decreased only by 1 log10 TCID50/mL in 3 min. However, the viral titre was decreased by more than 4 log10 TCID50/mL when treated with 80 ppm of each chemical for 10 s regardless of presence or absence of FBS. It should be emphasized that treatment with 24 ppm of ClO2 inactivated more than 99.99% SARS-CoV-2 within 10 s or 99.99% SARS-CoV-2 in 1 min in the presence of 0.5% or 1.0% FBS, respectively. By contrast, 24 ppm of sodium hypochlorite inactivated only 99% or 90% SARS-CoV-2 in 3 min under similar conditions. Notably, except for ClO2, the other components of Cleverin such as sodium chlorite, decaglycerol monolaurate, and silicone showed no significant antiviral activity.
Conclusion
Altogether, the results strongly suggest that although ClO2 and sodium hypochlorite are strong antiviral agents in absence of organic matter but in presence of organic matter, ClO2 is a more potent antiviral agent against SARS-CoV-2 than sodium hypochlorite.
Keywords: Chlorine dioxide, Sodium hypochlorite, SARS-CoV-2
Introduction
In December 2019, a new coronavirus suddenly emerged in Wuhan, China. This virus was isolated from patients with pneumonia and named as ‘severe acute respiratory syndrome coronavirus 2’ (SARS-CoV-2) [1]. The disease caused by this virus was termed COVID-19. SARS-CoV-2 quickly spread all over the world, and on March 11th, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic [2]. By June 30th, 2021, confirmed cases of, and deaths from, COVID-19 were >180 million, and almost four million, respectively; the disease remains a significant threat to mankind [3].
SARS-CoV-2 is transmitted through aerosols, droplets, and fomites [4]. Therefore, it is important to inactivate virus particles on surfaces contaminated by droplets from infected persons. WHO has recommended use of 70% ethanol or 1000 ppm of sodium hypochlorite (NaClO) for disinfection [5]. NaClO is likely the most commonly used surface disinfectant in the world. However, it is well known that antiviral activity of NaClO is readily decreased by the presence of organic matter such as saliva and blood [6].
Several studies have shown that 10 ppm of chlorine dioxide (ClO2) inactivated 99.999% of influenza A virus in the presence of 1% foetal bovine serum (FBS), whereas 100 ppm of NaClO, which is 10-fold higher concentration than that of ClO2, was required to inactivate the same degree of influenza A virus under the same conditions [7]. However, there is no evidence as to whether ClO2 inactivates SARS-CoV-2. If it does, there is no information available on how much ClO2, and for how long an exposure, is needed to inactivate SARS-CoV-2. Therefore, this study examined the antiviral activity of ClO2 against SARS-CoV-2 under various conditions in terms of its concentration, incubation time, and absence or presence of organic matter. The results were compared with those of NaClO.
Methods
Cell culture
VeroE6/TMPRSS2 cells were used for cultivation of SARS-CoV-2; the cells were incubated at 37°C in a 5% CO2 humidified incubator (ESPEC Corp., Osaka, Japan) [8]. VeroE6/TMPRSS2 cell line was purchased from the Japanese Collection of Research Bioresources (Osaka, Japan). The cells were cultured in Dulbecco's modified Eagle medium, low glucose, pyruvate (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 5% heat-inactivated foetal bovine serum (FBS; Thermo Fisher) and 1 mg/mL G418 (Nacalai Tesque, Inc., Kyoto, Japan).
Virus preparation
One hundred and forty thousand VeroE6/TMPRSS2 cells were cultured in a 25 cm2 cell culture flask at 37°C for 16 h in a 5% CO2 humidified incubator. The cells were infected with MOI = 0.001 of SARS-CoV-2 JPN/TY/WK-521 strain and incubated at 37°C in DMEM supplemented with 2% heat-inactivated FBS (Thermo Fisher) and 1 mg/mL of G418 (Nacalai Tesque) for 48 h. After a cytopathic effect had been observed, the spent culture medium was harvested and centrifuged at 1600 g for 5 min, and supernatant fraction containing virus particles was collected. One gram of polyethylene glycol 6000 and 233 mg of NaCl (Nacalai Tesque) were then added to 10 mL of collected virus solution and incubated at 4°C for 16 h. The virus solution was centrifuged at 20,000 g at 4°C for 10 min, the supernatant was discarded, and the pellet was suspended in 1 mL of PBS (–) at pH 7.4. Experiments with the live SARS-CoV-2 virus were conducted at the bio-safety level 3 laboratory in Osaka Prefecture University after obtaining the permission from the bio-risk committee of Osaka Prefecture University.
Antiviral activity of Cleverin, sodium hypochlorite, pure ClO2, sodium chlorite and Cleverin without ClO2 and sodium chlorite
Cleverin (Taiko Pharmaceutical Co. Ltd, Osaka, Japan) is a mixture of 500 ppm ClO2, 17,900 ppm sodium chlorite, 3300 ppm decaglycerol monolaurate, and 80 ppm silicone. Sixty microlitres of concentrated SARS-CoV-2 in PBS supplemented with 0, 2.5, or 5.0% FBS were mixed with 240 μL of diluted Cleverin containing several concentrations (1, 10, 30, or 100 ppm) of ClO2 or 1, 10, 30 or 100 ppm sodium hypochlorite (Wako pure chemical industries Ltd, Osaka, Japan), 10 or 100 ppm of pure ClO2 in which ClO2 gas is dissolved in ultrapure water, 6000 ppm of sodium chlorite or Cleverin without ClO2 and sodium chlorite (containing 660 ppm decaglycerol monolaurate and 16 ppm silicone). The mixture was then incubated at room temperature (25°C) for 10 or 30 s, and 1 or 3 min. After incubation, 540 μL of 5 mM sodium thiosulfate (Wako) was immediately added to 60 μL of the mixtures to neutralize the remaining activity of each chemical, after which 60 μL of 10 × DMEM (Nissui Pharmaceutical Co. Ltd, Tokyo, Japan), 12 μL of FBS and 12 μL of 50 mg/mL G418 disulfate aqueous solution were added. Subsequently, ten-fold dilution was carried out with DMEM supplemented with 2% FBS and 1 mg/mL G418 and titration was done as described below.
Titration of virus
Approximately 2.5 × 104 cells/100 μL of VeroE6/TMPRSS2 cells were seeded in a 96-well plate and cultured at 37°C for 16 h in the medium. Culture medium was removed and 100 μL of 10-fold serially diluted viral solution in DMEM supplemented with 2% FBS and 1 mg/mL G418 was added. The infected VeroE6/TMPRSS2 cells were cultured at 37°C for 72 h. Cells were fixed with methanol (Nacalai Tesque) and stained with 0.5% Crystal Violet. Fifty percent tissue culture infective dose per mL (TCID50/mL) was calculated. The detection limit was confirmed to be ≤2.2 log10 TCID50/mL.
Statistical analysis
Statistical analyses were performed using Microsoft Excel 2016 (Microsoft, Redmond, WA, USA). Error bars show standard deviations. P-values were calculated with Student's t-test using paired, two-tailed distribution. P < 0.05 was considered statistically significant.
Results
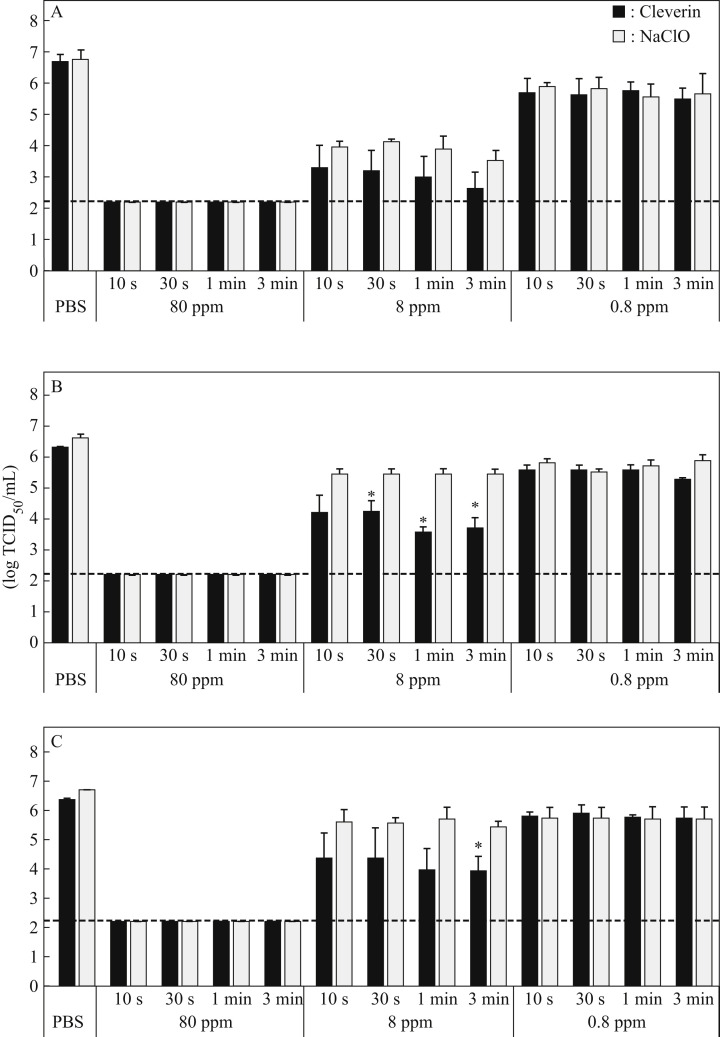
To examine and compare the antiviral activity of ClO2 in Cleverin against SARS-CoV-2 in the absence or presence of organic matter with that of sodium hypochlorite, the concentrated viruses suspended in PBS were treated with several concentrations of ClO2 or sodium hypochlorite in the absence or presence of FBS. Antiviral activity of both chemicals was observed in a concentration-dependent manner (Figure 1 ). When the viruses were treated with 80 ppm ClO2 or sodium hypochlorite as a final concentration regardless of presence of organic matter, the viral titre was decreased to the detection limit (≤2.2 log10 TCID50/mL) even in 10 s (Figure 1). When the viruses were treated with 8 ppm ClO2, the viral titre was decreased by 3–4 log10 TCID50 in the absence of organic matter (Figure 1A). In the case of 8 ppm sodium hypochlorite, the viral titre was decreased by 2–3 log10 TCID50 under the same conditions. However, there was no significant difference in viral titres between viruses treated with ClO2 and sodium hypochlorite (Figure 1A). In fact, 0.8 ppm of both chemicals decreased the viral titre by only 1 log10 TCID50 regardless of presence of organic matter.
Figure 1.
Antiviral activity of 0.8, 8, and 80 ppm of chlorine dioxide and sodium hypochlorite against SARS-CoV-2. Concentrated SARS-CoV-2 suspended in phosphate-buffered saline (PBS) without foetal bovine serum (FBS) (A), PBS with 0.5% FBS (B) or PBS with 1% FBS (C) was incubated with 80, 8, or 0.8 ppm of chlorine dioxide (ClO2) or sodium hypochlorite (NaClO) for 10 s, 30 s, 1 min, or 3 min as indicated. Viral titre was determined by measurement of 50% tissue culture infective dose per mL (TCID50/mL). All data represent the means ± SD from three independent experiments. Dashed line indicates the detection limit for each experiment. ∗Viral titre is significantly different between ClO2 and NaClO treatments.
When the viruses were treated with 8 ppm ClO2 in the presence of 0.5% FBS as final concentration, the viral titre was decreased by 2–3 log10 TCID50 whereas the titre was decreased by around 1 log10 TCID50 when treated with 8 ppm sodium hypochlorite under the same conditions (Figure 1B). Furthermore, when the viruses were treated with 8 ppm ClO2 for 30 s, 1 or 3 min in the presence of 0.5% FBS, the viral titre was significantly lower than when treated with 8 ppm sodium hypochlorite (Figure 1B). Similarly, when the viruses were treated with 8 ppm ClO2 in the presence of 1% FBS, the viral titre decreased by around 2–3 log10 TCID50 whereas it was decreased by only 1 log10 TCID50 when treated with 8 ppm sodium hypochlorite under the same conditions (Figure 1C). The viral titre was significantly lower when treated with 8 ppm ClO2 for 3 min compared with 8 ppm sodium hypochlorite (Figure 1C).
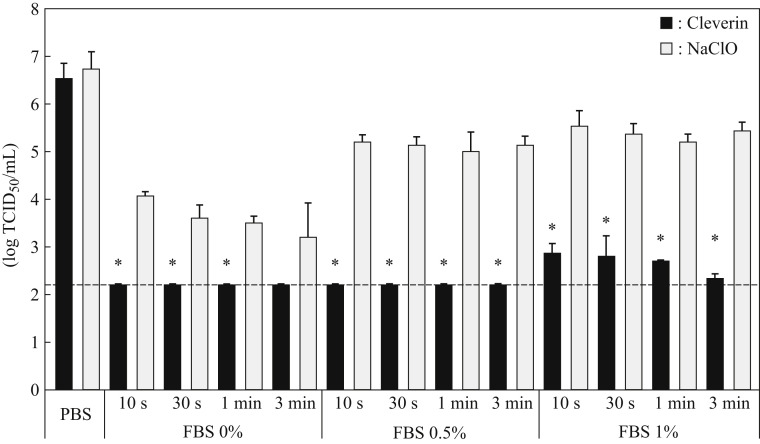
Since 80 ppm ClO2 inactivated the viruses completely but 8 ppm ClO2 inactivated partially, we further examined antiviral activity of 24 ppm ClO2 and the result was compared with that of sodium hypochlorite. When the viruses were treated with 24 ppm ClO2, the viral titre was decreased to below the detection limit (≤2.2 log10 TCID50/mL) even in the presence of 0.5% FBS in 10 s (Figure 2 ); however, the same concentration of sodium hypochlorite under the same conditions was unable to inactivate the viruses completely. Furthermore, when the viruses were treated with 24 ppm ClO2 in the presence of 1.0% FBS, the viral titre was decreased by about 4 log10 TCID50 even in 10 s and was significantly lower than that treated with the same concentration of sodium hypochlorite under the same conditions (Figure 2).
Figure 2.
Antiviral activity of 24 ppm chlorine dioxide or sodium hypochlorite against SARS-CoV-2. Concentrated SARS-CoV-2 suspended in phosphate-buffered saline (PBS) without foetal bovine serum (FBS), PBS with 0.5% FBS, or PBS with 1% FBS was incubated with 24 ppm chlorine dioxide (ClO2) or sodium hypochlorite (NaClO) for 10 s, 30 s, 1 min, or 3 min as indicated. Viral titre was determined by 50% tissue culture infective dose per mL (TCID50/mL). All data represent the means ± SD from three independent experiments. Dashed line indicates the detection limit for each experiment. ∗Viral titre is statistically different between ClO2 and NaClO treatments.
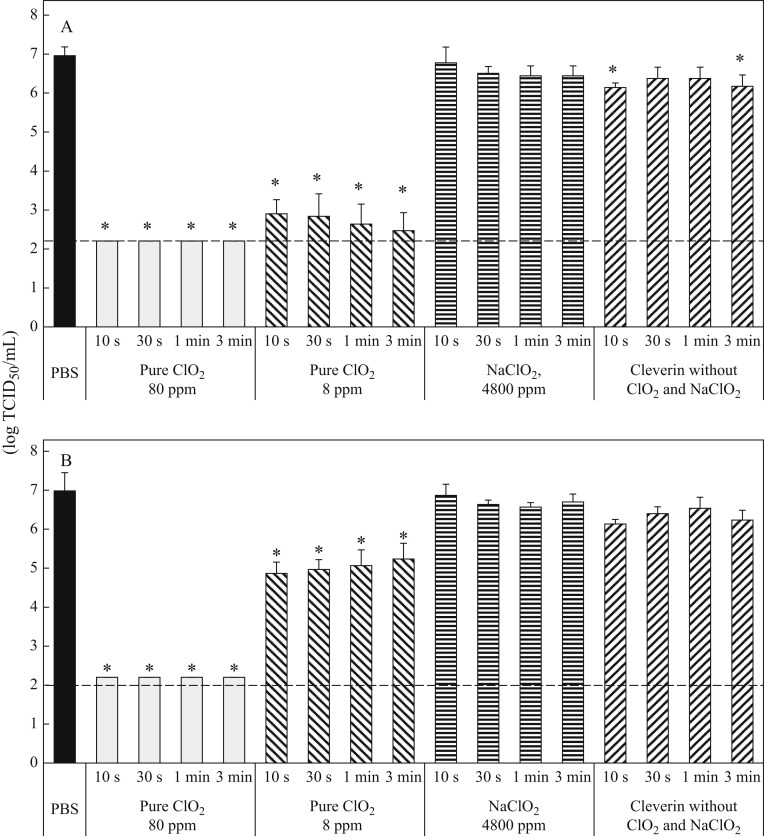
Cleverin is not pure ClO2: apart from ClO2 it also contains sodium chlorite, decaglycerol monolaurate, and silicone. Therefore, to confirm whether the antiviral activity of Cleverin is ClO2 dependent, the antiviral activity of pure ClO2, sodium chlorite, and mixture of decaglycerol monolaurate and silicone was further examined separately (Figure 3 ). When viruses were treated with 8 ppm of pure ClO2 the viral titre was decreased by >4 log10 TCID50 in the absence of FBS (Figure 3A) while the viral titre was decreased by about 2 log10 TCID50 only in 10 s in the presence of FBS (Figure 3B). However, when 80 ppm of pure ClO2 was applied, the viral titre was decreased to below the detection limit (≤2.2 log10 TCID50/mL) even in the presence of 1.0% FBS in 10 s (Figure 3B). On the other hand, 4800 ppm sodium chlorite was hardly able to decrease the viral titre and Cleverin devoid of 80 ppm ClO2 and 4800 ppm sodium chlorite but containing 528 ppm decaglycerol monolaurate, and 12.8 ppm silicone decreased viral titre only by 1 log10 TCID50 against SARS-CoV-2 regardless of presence or absence of FBS (Figure 3). Taken together, these data clearly indicate that the main component effecting the inactivation of SARS-CoV-2 is ClO2 but not the other components in Cleverin.
Figure 3.
Antiviral activity of 80 and 8 ppm pure chlorine dioxide, 4800 ppm of sodium chlorite and Cleverin without chlorine dioxide and sodium chlorite against SARS-CoV-2. Concentrated SARS-CoV-2, which was suspended in phosphate-buffered saline (PBS) without foetal bovine serum (FBS) (A) and with 1% FBS (B), respectively, was incubated with 80 or 8 ppm pure chlorine dioxide (ClO2), 4800 ppm sodium chlorite (NaClO2) or Cleverin without ClO2 and NaClO2 but containing 528 ppm of decaglycerol monolaurate and 12.8 ppm silicone for 10 s, 30 s, 1 min or 3 min. Viral titre was determined by measurement of 50% tissue culture infective dose per mL (TCID50/mL). All data represent the means ± SD from three independent experiments. Dashed line indicates detection limit for each test. ∗Viral titre is statistically different between PBS and each test.
Discussion
COVID-19 is an emerging disease and to date there is no highly effective treatment, although a few medicines have been found to improve clinical outcomes in large trials [9]. Rapid development and production of vaccines in several countries has permitted large-scale vaccination of subjects in many, mostly developed, countries [[10], [11], [12]]. However, the majority of the world's population remains unvaccinated, and it is uncertain how effective vaccination will be in the longer term as immunity wanes, and new SARS-CoV-2 variants emerge [13,14]. Thus disinfectants active against SARS-CoV-2 will remain a cornerstone of control of COVID-19 globally. Sodium hypochlorite is one of the most popular disinfectants in clinical settings. However, sodium hypochlorite has some disadvantages; for example, it may produce more trihalomethane and it exhibits weak antimicrobial activity in presence of organic matters compared to ClO2 [7,15,16]. Therefore, our study tested antiviral activity of ClO2, which is a major component of Cleverin, against SARS-CoV-2.
Eighty parts per million of both ClO2 and sodium hypochlorite inactivated 6–7 log10 TCID50/mL of SARS-CoV-2 to below the detection limit (≤2.2 log10 TCID50/mL) in just 10 s even in the presence of 1.0% FBS (Figure 1C), indicating that both ClO2 and sodium hypochlorite may be useful disinfectants against SARS-CoV-2 in saliva. Saliva is the most important infection source and contains ∼1.1 mg/mL proteins [17]. In this study, 5.0% FBS was used as highest concentration in virus solution (1.0% FBS as final concentration), which is two times higher in protein concentration than that in saliva.
Further, the study examined the antiviral activity of pure ClO2, sodium chlorite, and Cleverin without ClO2 and sodium chlorite independently, since Cleverin, in addition to 100 ppm ClO2, also contains 6000 ppm sodium chlorite, 660 ppm decaglycerol monolaurate, and 16 ppm silicone. In fact, 60 μL of 100 ppm ClO2 (80 ppm, a final concentration) showed the same antiviral activity with Cleverin, but 60 μL of 6000 ppm sodium chlorite (4800 ppm, a final concentration), a precursor of ClO2, showed no antiviral activity. In addition, 60 μL mixture of 660 ppm decaglycerol monolaurate (528 ppm, a final concentration) and 16 ppm silicone (12.8 ppm, a final concentration) was also tested and showed no significant reduction of viral titre in the presence of 1.0% FBS. However, viral titre was slightly decreased (∼1 log10 TCID50 reduction) when FBS was absent (Figure 3). Since decaglycerol monolaurate is a surfactant, this compound might affect the envelope or proteins of SARS-CoV-2 [18]. Nevertheless, we conclude that the antiviral activity of Cleverin is ClO2 dependent.
When 24 ppm sodium hypochlorite was exposed to the virus, the viral titre decreased by 4 log10 TCID50 in 30 s in the absence of FBS (Figure 2); however, when 0.5% or 1.0% FBS was present, 24 ppm sodium hypochlorite reduced the viral titre by only 2 log10 TCID50 even in 3 min. It should be noted that 24 ppm of ClO2 reduced the viral titre to below the detection limit (≤2.2 log10 TCID50/mL) even in 10 s in the presence of 0.5% FBS and by >4 log10 TCID50 in 30 s in the presence of 1.0% FBS (Figure 2), suggesting that ClO2 is a much more powerful disinfectant than sodium hypochlorite, especially when organic matter is present in the contaminants. This advantage was also demonstrated by other pathogens such as influenza A virus and multidrug-resistant (MDR) bacteria such as meticillin-resistant Staphylococcus aureus (MRSA), MDR Pseudomonas aeruginosa (MDRP) and MDR Acinetobacter baumannii (MDRA) [7,16]. ClO2 has been shown to have 10-fold higher antiviral activity than sodium hypochlorite against influenza A virus in the presence of 1% FBS [7]. When the virus was exposed to ClO2, their major surface glycoproteins such as haemagglutinin and neuraminidase, responsible for the viral infection to and release from cells, were degraded [19]. This could be the mechanism by which ClO2 inactivates virus infectivity. Other examples are MDR bacteria: ClO2 had more effective antimicrobial activity than sodium hypochlorite against MRSA, MDRP, and MDRA in the presence of 3.0% BSA and 3.0% sheep erythrocyte [16]. In addition, ClO2 is less toxic than sodium hypochlorite because of production of the carcinogen trihalomethane by the latter [15]. Taken together, these observations might point to ClO2 being more useful than sodium hypochlorite to inactivate SARS-CoV-2, especially in clinical material.
Antiviral activity of ClO2 against SARS-CoV-2 was expected because it has been recently demonstrated that ClO2 may inhibit binding of recombinant spike protein of SARS-CoV-2 to its receptor molecule, angiotensin-converting enzyme 2 (ACE-2) [20]. It has also been demonstrated that ClO2 can denature proteins by oxidative modification of tryptophan and tyrosine residues [21]. Since tyrosine at position 453, which is located in the receptor-binding domain of the spike protein, forms a hydrogen bond with histidine at position 34, located in an alpha 1 helix of the ACE-2 protein, oxidative modification of the tyrosine by ClO2 could reduce the infectivity of the virus [22]. Indeed, infectivity of SARS-CoV-2 to the VeroE6/TMPRSS2 cells has been demonstrated to be significantly reduced by ClO2 in this study.
Various mutant strains of SARS-CoV-2 have emerged in the UK, South Africa, and Brazil, and have spread in many countries throughout the globe [13,14]. These strains have a mutation in asparagine at position 501 to tyrosine (N501Y) in the spike protein of SARS-CoV-2, which is also responsible for receptor binding. Two new SARS-CoV-2 lineages (N501Y) reported in the UK are more transmissible than the 501N lineage. Since ClO2 targets tryptophan and tyrosine in proteins, ClO2 might inactivate these novel mutants efficiently. Currently experiments are under way in our laboratory to verify whether ClO2 can inactivate these mutant viral strains as effectively as in the case of the wild-type strain.
In conclusion, our data indicate that ClO2 is a more effective disinfectant against SARS-CoV-2 than NaClO in presence of organic matter. Use of 24 ppm ClO2 inactivated 6.5 log10 TCID50/mL of SARS-CoV-2 to below the detection limit even in the presence of 0.5% FBS in 10 s. Therefore, ClO2 is a powerful disinfectant against SARS-CoV-2 and it may be useful for the reduction of SARS-CoV-2 infection.
Acknowledgements
We thank the National Institute of Infectious Diseases (Tokyo, Japan) for providing the SARS-CoV-2 JPN/TY/WK-521 strain. We thank Dr Rupak K. Bhadra, CSIR-Indian Institute of Chemical Biology, Kolkata, India, for critically reading the manuscript.
Conflict of interest statement
This study was performed as a collaborative research of Taiko Pharmaceutical Co. Ltd.
Funding sources
Supported by Taiko Pharmaceutical Co. Ltd.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Archived: WHO timeline-COVID-19. Available at: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 [last accessed June 2021].
- 3.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/ [last accessed June 2021].
- 4.Kumar G.D., Mishra A., Dunn L., Townsend A., Oguadinma I.C., Bright K.R., et al. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front Microbiol. 2020;11:1351. doi: 10.3389/fmicb.2020.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Cleaning and disinfection of environmental surfaces in the context of COVID-19. Available at: https://www.who.int/publications/i/item/cleaning-and-disinfection-of-environmental-surfaces-inthe-context-of-covid-19 [last accessed September 2021].
- 6.Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11:147–157. doi: 10.4265/bio.11.147. [DOI] [PubMed] [Google Scholar]
- 7.Miura T., Shibata T. Antiviral effect of chlorine dioxide against influenza virus and its application for infection control. Open Antimicrob Agents J. 2010;2:71–78. [Google Scholar]
- 8.Matsuyama S., Nao N., Shirota K., Kawase M., Saito S., Takayama I., et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2 expressing cells. Proc Natl Acad Sci USA. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1237 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 12.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomized, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung K., Shum M.H.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021;26:2002106. doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makoni M. South Africa responds to new SARS-CoV-2 variant. Lancet. 2021;397:267. doi: 10.1016/S0140-6736(21)00144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorlini S., Collivignarelli C. Trihalomethane formation during chemical oxidation with chlorine, chlorine dioxide and ozone of ten Italian natural waters. Desalination. 2005;176:103–111. [Google Scholar]
- 16.Hinenoya A., Awasthi S.P., Yasuda N., Shima A., Morino H., Koizumi T., et al. Chlorine dioxide is a better disinfectant than sodium hypochlorite against multi-drug resistant Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii. Jpn J Infect Dis. 2015;68:276–279. doi: 10.7883/yoken.JJID.2014.294. [DOI] [PubMed] [Google Scholar]
- 17.Jenzano J.W., Hogan S.L., Noyes C.M., Featherstone G.L., Lundblad R.L., et al. Comparison of five techniques for the determination of protein content in mixed human saliva. Anal Biochem. 1986;159:370–376. doi: 10.1016/0003-2697(86)90355-6. [DOI] [PubMed] [Google Scholar]
- 18.Simon M., Veit M., Ostrrieder K., Gradzielski M. Surfactants – compounds for inactivation of SARS-CoV-2 and other enveloped viruses. Curr Opin Colloid Interface Sci. 2021;55:101409. doi: 10.1016/j.cocis.2021.101479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata T., Ogata N. Protective effect of low-concentration chlorine dioxide gas against influenza A virus infection. J Gen Virol. 2008;89:60–67. doi: 10.1099/vir.0.83393-0. [DOI] [PubMed] [Google Scholar]
- 20.Ogata N., Miura T. Inhibition of the binding of spike protein of SARS-CoV-2 coronavirus to human angiotensin-converting enzyme 2 by chlorine dioxide. Ann Pharmacol Pharmaceut. 2020;5:1195. [Google Scholar]
- 21.Ogata N. Denaturation of protein by chlorine dioxide: oxidative modification of tryptophan and tyrosine residues. Biochemistry. 2007;46:4898–4911. doi: 10.1021/bi061827u. [DOI] [PubMed] [Google Scholar]
- 22.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]