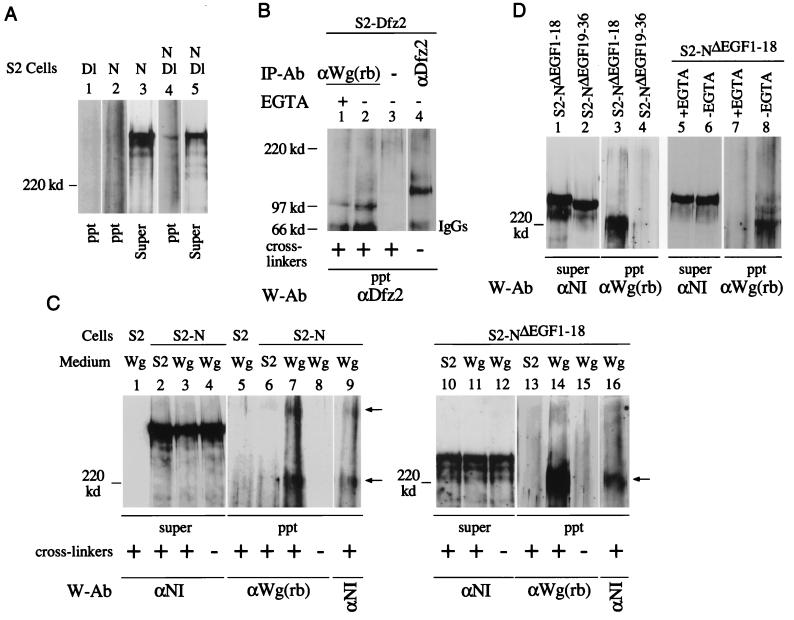
FIG. 3.
Two different Wg- and N-containing complexes are recovered from N-expressing S2 cell surfaces. (A) N- and Dl-containing complexes are recovered from S2-N and S2-Dl cell aggregates in the presence of cross-linkers. Dl-containing cross-linked complexes were immunoprecipitated by the monoclonal anti-Dl antibody, MAb 202 (24) and analyzed by Western blotting with anti-NI antibody. (B) Wg- and Dfz2-containing cross-linked complexes are recovered from S2-Dfz2 cells treated with Wg medium containing cross-linkers, in the presence or absence of EGTA (lanes 1 and 2). A mouse monoclonal anti-Dfz2 antibody (kindly provided by R. Nusse) was used for immunoprecipitation (lane 4) and for detection of Wg-Dfz2 complexes by Western blotting. Wg-Dfz2 complexes were not recovered from S2-Dfz2 cells treated with medium conditioned by growth of S2 cells (not shown). (C) Two Wg- and N-containing cross-linked complexes (arrows) are immunoprecipitated from S2-N cells (lanes 7 and 9), and only one is immunoprecipitated from S2-NΔEGF1–18 cells (lanes 14 and 16), treated with Wg-containing medium. N- and Wg-containing complexes were immunoprecipitated with anti-Wg(rb) and detected by Western blotting with the indicated antibodies (W-Ab). Lanes 7 and 9 and lanes 14 and 16 show reaction of the same blots with anti-Wg(rb) and anti-NI antibodies. (D) Wg and N containing cross-linked complexes are recovered from S2-NΔEGF1–18 cells (lane 3) in the absence of EGTA (lane 8) but not from S2-NΔEGF19–36 cells (lane 4). S2-NΔEGF1–18 or S2-NΔEGF19–36 cells were treated with Wg medium containing cross-linkers, immunoprecipitation was performed with anti-Wg(rb) antibody, and the Western blots were probed with the indicated antibodies. ppt, immunoprecipitated complexes eluted from GammaBind beads; Super, an aliquot of the protein extract after the last pelleting of the GammaBind beads (see Materials and Methods); IP-Ab, immunoprecipitation antibody; W-Ab, Western blotting antibody; cross-linker, BS3. Wg, medium conditioned by growth of S2-Wg cells; S2, medium conditioned by growth of S2 cells. For panels A, C, and D, 4% polyacrylamide gels were used; for panel B, 6% polyacrylamide gels were used. Only proteins or protein complexes migrating slower than a 120-kDa marker protein are resolved in panels A, C, and D. The tops of all the blots shown coincide with the top of the resolving gel of the discontinuous SDS-PAGE gels.