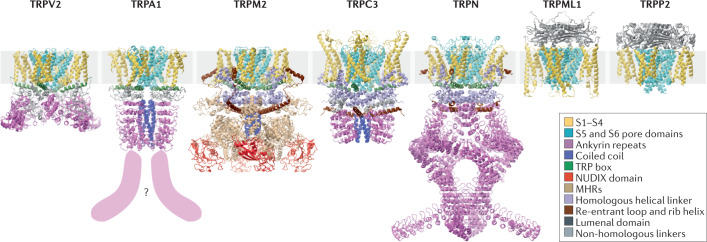
Fig. 1. Similarities and differences between the structures of TRP channels.
Representative structures for each TRP channel subfamily, coloured to highlight common structural features. The S1−S4 (gold) and the S5 and S6 pore domains (cyan) are the only domains common to all subfamilies. The TRPA, TRPV, TRPM, TRPC and TRPN channels have TRP box helices (dark green). The TRPA, TRPC, TRPN and TRPV channels have amino (N)-terminal cytoplasmic ankyrin repeats (violet; the TRPA1 structure is missing about 11 repeats, as indicated by the question mark and violet shapes). The TRPC, TRPM and TRPN channels have a homologous pre-S1 helical linker (lilac) and C-terminal re-entrant loop and rib helix (brown), which is followed by a coiled coil in the TRPC and TRPM channels (blue; TRPA channels also have a carboxy (C)-terminal coiled coil). The melastatin homology regions (MHRs) of TRPM channels are shown in tan, and the NUDIX domain unique to TRPM2 is shown in red. The TRPML and TRPP channels have a homologous lumenal domain (dark grey). The pre-S1 linkers of the TRPV and TRPA channels and the C-terminal region of the TRPV channels (light grey) share no clear homology outside their respective subfamilies. The structures depicted are human TRPA1 (PDB ID 3J9P), human TRPC3 (PDB ID 6CUD), human TRPM2 (PDB ID 6MIX), Drosophila TRPN (NompC; PDB ID 5VKQ), human TRPP2 (PDB ID 5T4D), human TRPML1 (PDB ID 5JW5) and rabbit TRPV2 (PDB ID 6OO3).