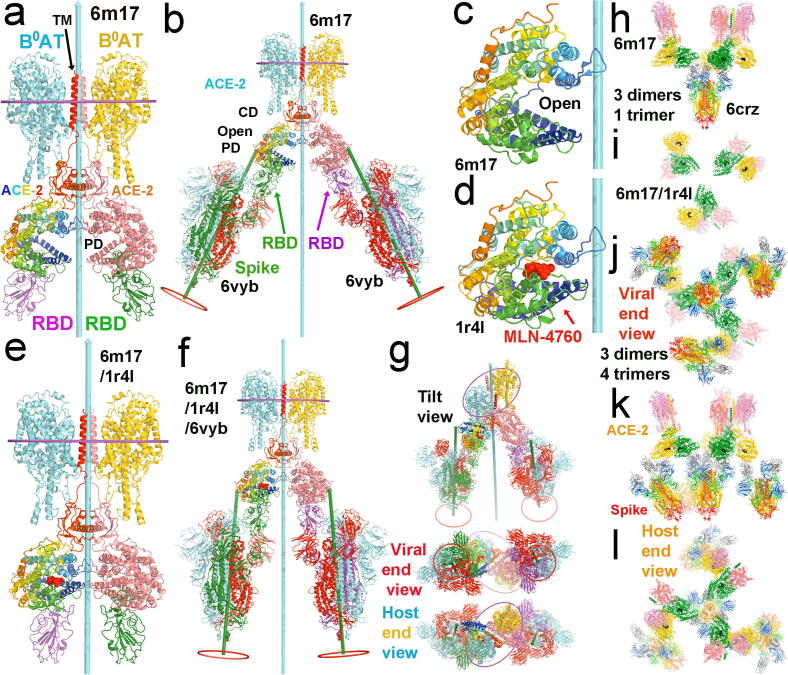
Fig. 3.
The full-length ACE2 dimers in complex with the two RBDs of spike proteins and with two neutral amino-acid transporter B0AT for modeling super-complexes. (a) Dimer of ACE2/B0AT heterodimer (PDB ID/6m17). One ACE2 is rainbow colored from N-to-C termini, and the other is salmon. One B0AT is in cyan, and the other is in yellow. Two RBDs of spike are in magenta and cyan. (b) Docking two asymmetric spike trimers (6vyb) onto the 6m17 ACE2 dimer by aligning their RBD-PD interaction. The dyad is in cyan with a length of 400 Å, and the 3-fold axes are in green with a length of 250 Å. Middle planes of the host membrane are indicated with ellipses surrounding the TMDs of the ACE2/B0AT dimer; locations of the viral membrane are shown red circles, which differ between the two spike trimers, and between the spike trimers and the ACE2 dimer. Distance between the two spike trimers at the viral membrane is about 260 Å. (c) A close-up view of the PD of ACE2 in 6m17. (d) A close-up view of the testicular ACE2 PD with the inhibitor MLN-4760 (red spheres) bound in the closed conformation (1r4l). (e) Modeled closed ACE2 on the basis of 6 m17 and 1r4l. (f) Docking two asymmetric spike trimers (6vyb) onto the closed ACE2 dimer. Spacing between the two spike trimers is about 140 Å. (g) Three additional views of (f), tilted view, viral end view, and host end view. (h) One symmetrized 6crz spike trimer with three closed ACE2 dimers. (i) The 3-fold arrangement of the three closed ACE2 dimers viewed from the viral end with the 6crz trimer removed. (j-l) Three views of the smallest function of spike/ACE2 super-complex, containing three ACE2 dimers and three spike trimers surrounding the central spike trimer. (j) Viral end view. (k) Side view with viral membrane the bottom and host membrane on the top (l) Host end view. See Video 2A and 2B for opening and closing of the ACE2 cleft in complexes with the spike trimers with and without an inhibitor of ACE2 bound. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)