Product scope for the Cu-mediated 18F-fluorination of aryl-tetrazines starting from stannane precursors.
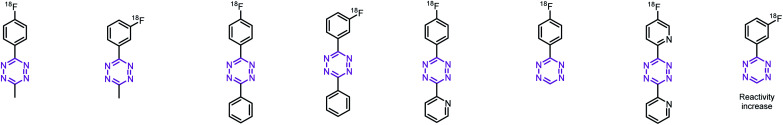
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | [18F]6 | [18F]7 | [18F]8 | [18F]9 | [18F]10 | [18F]11 | [18F]12 | [18F]13 |
| RCCa [%] | 30 ± 5 | 28 ± 1 | 30 ± 5 | 31 ± 2 | —d | 18 ± 4 | —d | 12 ± 1 |
| RCYb [%] | 23 ± 1 | 26 ± 2 | 23 ± 2 | 24 ± 3 | —d | 15 ± 3 | —d | 11 ± 3 |
| Rel. reactivityc | 1.0 | 1.4 | 1.8 | 3.0 | 10 | 70 | 91 | 96 |
| RCPa [%] | ≥99 | ≥99 | ≥99 | ≥99 | —d | 99 | —d | 99 |
Radiochemical conversion (RCC) and radiochemical purity (RCP) were determined by radio-HPLC and radio-TLC (n = 3).
Radiochemical yield (RCY) was decay corrected to the starting amount of radioactivity received from the cyclotron and the isolated product without a formulation step (n = 3).
Relative IEDDA reactivity was calculated based on second order rate constants determined by stopped-flow measurements of the respective reference compound (19F-Tz) with trans-cyclooctene at 25 °C in 1,4-dioxane or acetonitrile (see the ESI).
No product could be isolated.
