Abstract
Background
To evaluate the frequency and associated characteristics of chronic comorbid conditions and obstetrical complications among pregnant women with human immunodeficiency virus (HIV) and receiving antiretroviral therapy (ART) in comparison to those without HIV.
Methods
We compared 2 independent concurrent US pregnancy cohorts: (1) with HIV (International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025, 2002–2013) and (2) without HIV (Consortium for Safe Labor Study, 2002–2007). Outcomes were ≥2 chronic comorbid conditions and obstetrical complications. For women with HIV, we assessed whether late prenatal care (≥14 weeks), starting ART in an earlier era (2002–2008), and a detectable viral load at delivery (≥400 copies/mL) were associated with study outcomes.
Results
We assessed 2868 deliveries (n = 2574 women) with HIV and receiving ART and 211 910 deliveries (n = 193 170 women) without HIV. Women with HIV were more likely to have ≥2 chronic comorbid conditions versus those without HIV (10 vs 3%; adjusted OR [AOR]: 2.96; 95% CI: 2.58–3.41). Women with HIV were slightly less likely to have obstetrical complications versus those without HIV (both 17%; AOR: .84; 95% CI: .75–.94), but secondarily, higher odds of preterm birth <37 weeks. Late entry to prenatal care and starting ART in an earlier era were associated with a lower likelihood of ≥2 chronic comorbidities and obstetrical complications; detectable viral load at delivery was associated with a higher likelihood of obstetric complications.
Conclusions
Pregnant women with HIV receiving ART have more chronic comorbid conditions, but not necessarily obstetrical complications, than their peers without HIV.
Keywords: HIV, comorbid conditions, comorbidities, morbidity, pregnancy
Pregnant women with human immunodeficiency virus (HIV) and receiving antiretroviral therapy have more chronic comorbid conditions, but not obstetrical complications, compared with those without HIV.
Individuals with human immunodeficiency virus (HIV) are living longer due to increasingly effective antiretroviral therapy (ART), resulting in a growing recognition that they have more chronic comorbid conditions compared with their counterparts without HIV [1–3]. This burden of noninfectious chronic comorbidities and related costs for individuals with HIV is rising in the United States [4]. In pregnancy, the prevalence of chronic comorbid conditions has steadily increased over time, regardless of HIV infection status, including hypertension [5], substance use and psychiatric illness [6], diabetes [7], heart disease [8], and liver disease [9]. Multiple chronic comorbid conditions increase the risk of maternal morbidity [10], as well as obstetrical complications [11]. Whether US pregnant women with HIV and receiving ART have more chronic comorbid conditions and obstetrical complications compared with their pregnant peers without HIV remains to be defined [12, 13].
Every year approximately 8500 women with HIV deliver in the United States, and who overwhelmingly (>80%) are receiving ART and with an undetectable plasma viral load [14, 15]. Pregnant women with HIV are more likely to be of Black race and lower socioeconomic status [16], as well as experience more fragmented and substandard prenatal and HIV care [17], which together may amplify the risk of adverse outcomes. Antiretroviral therapy decreases HIV-related and -unrelated complications for pregnant women [18–21]. Women with HIV experience more medically complex pregnancy hospitalizations compared with those without HIV [22], as well as more obstetrical complications, including growing concern that ART exposure increases the risk of preterm birth [23–26]. Understanding the relative burden of chronic comorbid conditions and obstetrical complications among pregnant women with HIV on ART can inform pregnancy care for this high-risk population [27, 28].
We evaluated the frequency and associated characteristics of chronic comorbid conditions and obstetrical complications among pregnant women with HIV and receiving ART and compared them with their counterparts without HIV infection.
METHODS
Study Setting
We conducted a secondary analysis of 2 large, independent, but concurrent, cohort studies of deliveries to US pregnant women with and without HIV. Pregnant women with HIV were enrolled in the International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025 conducted from 2002 to 2013 (hereafter referred to as IMPAACT P1025). Pregnant women without HIV were from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Consortium on Safe Labor conducted from 2002 to 2007 (hereafter referred to as CSL). Of note, less than 1% of deliveries during the study period were from 2007 in the current analysis. The original CSL study included data through 2007 (26% of deliveries in the original study) and 0.1% in 2008, but most deliveries after 2007 were not included in the current analysis due to unknown HIV status. For IMPAACT P1025, institutional review board approval was obtained at all clinical sites, and written informed consent was obtained from all enrolled women; for CSL, a de-identified dataset under a waiver of informed consent was approved by the Institutional Review Board at The Ohio State University.
Briefly, IMPAACT P1025 was a prospective cohort of deliveries with HIV at 20 weeks or more of gestation across 56 centers in the United States and Puerto Rico [16, 29]. The CSL study included deliveries at 23 weeks or more of gestation at 19 hospitals across the United States. Data linkage, cleaning, recording, and validation have been previously described for both studies [29, 30]. Data extracted from the maternal medical record and linked infant record included demographics, prenatal complications, delivery information, and maternal and neonatal outcomes.
Study Participants
We included women enrolled in IMPAACT P1025 or CSL who delivered at 23 weeks or more of gestation. For IMPAACT P1025, an additional criterion for inclusion was receiving ART at delivery, defined as 3 or more antiretroviral drugs (ARVs) from any class including 3 or more ARVs with a protease inhibitor, 3 or more ARVs with nonnucleoside reverse transcriptase inhibitors, or 3 or more nucleoside reverse transcriptase inhibitors (NRTIs). For CSL, an additional criterion for inclusion was no documented HIV infection at delivery, and those with HIV or unknown status were excluded because information about their HIV care was not available and patient overlap with IMPAACT P1025 was possible. Consistent with prior analyses from both datasets, we included women with multiple deliveries during the study time period (10% for IMPAACT P1025 and 9% for CSL), and all models adjusted for interpersonal correlation (ie, maternal clusters) [20, 31].
Exposures, Outcomes, and Covariates
The primary outcome was 2 or more chronic comorbid conditions, as defined by the World Health Organization and the UK National Institute for Health and Care Excellence (NICE) guidelines [32, 33], and obstetrical complications, consistent with the recent core outcome set for immunomodulation in pregnancy [34]. Secondarily, given the distinct pathophysiology for included conditions and the continued lack of standardization of multimorbidity across studies [35, 36], we assessed chronic comorbidities by primary disease system, including the following: (1) cardiovascular, (2) pulmonary, (3) metabolic, (4) hematologic, (5) neurological, (6) psychiatric, and (7) other, as well as any chronic comorbidity versus none [37]. We also assessed specific obstetrical and infant complications assessed in the composite obstetrical outcome, including (1) very preterm birth (<34 weeks’ gestation), (2) any preeclampsia, (3) gestational diabetes, (4) fetal growth restriction, (5) gestational hypertension, and (6) other (Supplementary Table 1). For IMPAACT P1025, outcomes were manually abstracted from the maternal and neonatal chart by trained study nurses and were then confirmed by 2 study maternal-fetal medicine specialists (K. K. V. and E. G. L.) [29]. For the CSL, multiple strategies were used to ascertain outcomes as previously described, including review of the electronic record, International Classification of Diseases, Ninth Edition (ICD-9), coding, and manual chart review with validation of some diagnoses [38].
The primary exposure was HIV infection. Then, among women with HIV, we assessed whether (1) timing of initiation of prenatal care (≥14 weeks’ vs <14 weeks’ gestation), (2) era when delivery occurred (2002–2008, an era of less-effective therapy during pregnancy when ART was not universally recommended for all pregnant women as previously defined by our group, vs 2009–2013), and (3) viral load at delivery (≥400 copies/mL vs <400 copies/mL) were associated with chronic comorbidities and obstetrical complications [20].
Statistical Analysis
We compared chronic comorbid conditions and obstetrical complications among deliveries to pregnant women with and without HIV. We fit multivariable logistic regression models with generalized estimating equations, accounting for multiple deliveries from the same woman during the study periods for both cohorts. We assessed patient characteristics for confounding a priori by constructing a directed acyclic graph [39]. For the first model comparing women with versus without HIV, we adjusted for maternal age, race/ethnicity, parity, delivery year, and geographic region. For the outcome of obstetrical complications, we also adjusted for multiple gestation and history of prior preterm birth. For the second model among women with HIV, we adjusted for the above variables as well as initial CD4 cell count in pregnancy, HIV viral load suppression at delivery, ART guideline period, and timing of initiation of prenatal care. For covariates with missing values, multiple imputation was used to assess the effect of missing data. We conducted 3 sensitivity analyses: (1) given the possibility of outcome misclassification between both studies, we assessed whether the primary analysis held comparing women with known HIV infection with those without restricted to the CSL (of note, ART exposure was not available); (2) given the possibility of changing trends of study outcomes during the non-concurrent time period between both studies (ie, after 2006 through 2013, deliveries were to women with HIV from IMPAACT P1025), we compared the frequency of study outcomes between the 2 time periods restricted to IMPAACT P1025 (2002–2006 and 2007–2013); and (3) we excluded women with an antiretroviral regimen consisting of 3 or more NRTIs to assess whether the primary analysis held for those receiving ART of at least 2 drug classes. All analyses used SAS version 9.4 software (SAS Institute, Inc, Cary, NC).
RESULTS
Among 3088 deliveries to 2748 women with HIV enrolled in IMPAACT P1025, 220 (7%) deliveries occurred without documented ART at the time of delivery and were excluded. Out of 228 438 deliveries to 208 695 women in the CSL, 902 (0.4%) deliveries occurred among women with HIV and 15 627 (6.8%) occurred among women with unknown HIV infection status, and both groups were excluded. Thus, the final study sample consisted of 2868/3088 or 93% of deliveries (n = 2574 women) with HIV and receiving ART in IMPAACT P1025 and 211 910/228 438 or 93% of deliveries (n = 193 170 women) without HIV in CSL.
Women with HIV were more likely to be parous compared with those without HIV, be of Black race, reside in the South, and have a history of prior preterm birth (Table 1). More also had a cesarean delivery compared with those without HIV. Among deliveries to women with HIV, 66% enrolled in prenatal care at less than 14 weeks’ gestation, 84% had suppressed viral load at delivery, and 53% initiated ART between 2002 and 2008 (Table 1). The most common antiretroviral regimen included a protease inhibitor (81%).
Table 1.
Characteristics of Deliveries to Women With and Without Human Immunodeficiency Virus
| Characteristics | Deliveries to Women With HIV in IMPAACT P1025a (n = 2868) (Column Percentage) | Deliveries to Women Without HIV in CSLb (n = 211 910) (Column Percentage) | P c |
|---|---|---|---|
| Maternal age at delivery, mean (SD), years | 28.2 (6.1) | 27.8 (6.2) | .002 |
| Parity | <.001 | ||
| 0 | 493 (17) | 84 924 (40) | |
| 1 or more | 2375 (83) | 126 986 (60) | |
| Delivery year | <.001 | ||
| 2002 | 149 (5) | 16 952 (8) | |
| 2003 | 219 (8) | 15 522 (7) | |
| 2004 | 281 (10) | 58 428 (28) | |
| 2005 | 328 (11) | 66 683 (31) | |
| 2006 | 246 (9) | 53 977 (25) | |
| 2007 | 294 (10) | 290 (<1) | |
| 2009 | 326 (11) | … | |
| 2010 | 289 (10) | … | |
| 2011 | 288 (10) | … | |
| 2012 | 300 (10) | … | |
| 2013 | 148 (5) | … | |
| Maternal race | <.001 | ||
| Black | 1671 (58) | 46 374 (23) | |
| White | 259 (9) | 103 654 (51) | |
| Latina | 857 (30) | 38 406 (19) | |
| Other | 81 (3) | 14 218 (7) | |
| US Census region | <.001 | ||
| Northeast | 685 (24) | 55 645 (26) | |
| West | 719 (25) | 68 630 (32) | |
| Midwest | 308 (11) | 18 928 (9) | |
| South, including Puerto Rico | 1156 (41) | 68 707 (32) | |
| Mode of delivery | <.001 | ||
| Vaginal | 1365 (48) | 151 079 (71) | |
| Cesarean | 1467 (52) | 60 830 (29) | |
| Gestational age at delivery, mean (SD), weeks | 38.2 (2.2) | 38.2 (2.5) | <.001 |
| History of preterm birth | 414 (14) | 15 372 (7) | <.001 |
| Multiple gestation | 51 (2) | 4746 (2) | .09 |
| Among women with HIV in IMPAACT P1025 | |||
| Entry into prenatal care | … | … | |
| ≥14 weeks | 985 (34) | ||
| <14 weeks | 1874 (66) | ||
| Viral load at delivery | … | … | |
| ≥400 copies/mL | 438 (16) | ||
| <400 copies/mL | 2384 (84) | ||
| ART guideline period | … | … | |
| 2002–2008 | 1517 (53) | ||
| 2009–2013 | 1351 (47) | ||
| ART regimen | … | … | |
| ≥3 NRTIs | 338 (12) | ||
| ≥3 ARVs with NRTI | 196 (7) | ||
| ≥3 ARVs with PI | 2334 (81) |
Data are presented as n (%) unless otherwise indicated. Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral drug; CSL, Consortium on Safe Labor; HIV, human immunodeficiency virus; IMPAACT P1025, International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
aMissing data in IMPAACT P1025 for the following covariates: gestational age at delivery (n = 8), history of preterm birth (n = 7), entry into prenatal care (n = 9), RNA viral load at delivery (n = 46), mode of delivery (n = 36),
bMissing data in CSL for the following covariates: age at delivery (n = 321), delivery year (n = 58), race (n = 9258), history of preterm birth (n = 5374), and mode of delivery (n = 1).
cA statistically significant P value <.05 may reflect a difference due to large sample size.
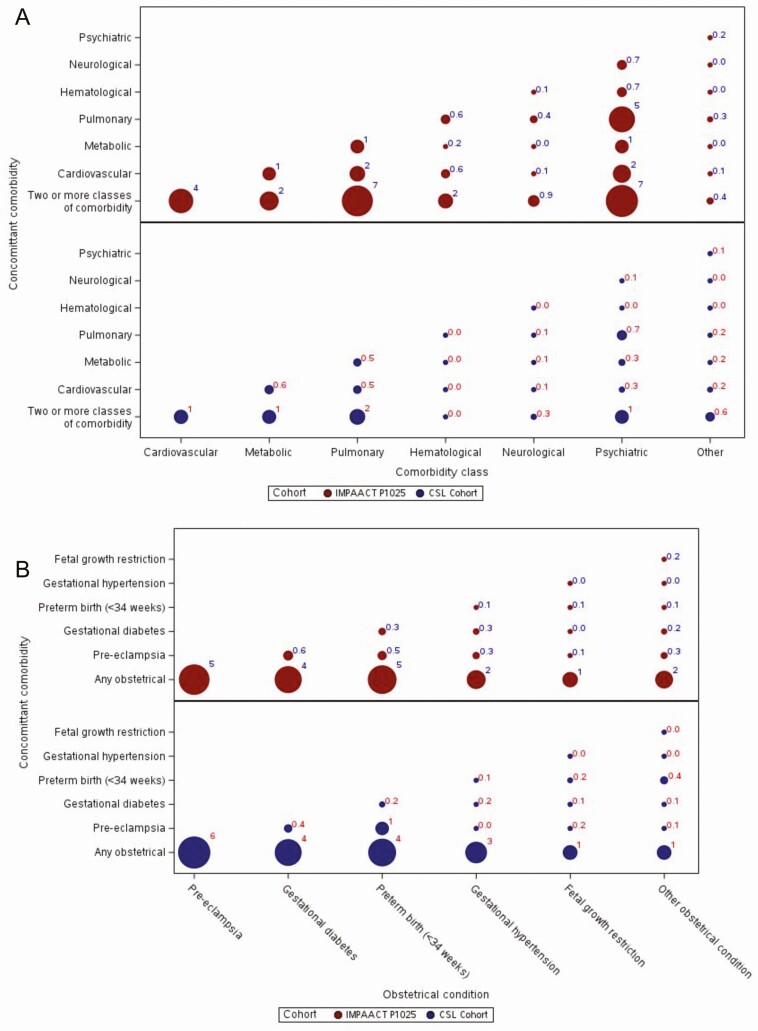
One in three deliveries with HIV (33%) had a chronic comorbid condition, compared with 1 in 5 without HIV (20%). Among deliveries to women with and without HIV, when assessing for trends over time, the frequency of 2 or more chronic comorbid conditions as well as obstetrical complications did not change over the respective study periods. As demonstrated by bubble plots, in which the size of the bubble corresponds to the frequency of the outcome, chronic comorbid conditions frequently occurred together but more so with HIV (Figure 1A). Obstetrical complications also frequently occurred together but did not vary by HIV infection status (Figure 1B).
Figure 1.
A, Bubble plot of relationship between chronic comorbid conditions for deliveries to women with (IMPAACT P1025) and without (CSL) HIV. B, Bubble plot of relationship between obstetrical conditions among women with (IMPAACT P1025) and without (CSL) HIV. All values are percentages. Values ≥1.0 have been rounded to the nearest whole number, while values <1.0 have been rounded to the nearest tenth (0.1). Abbreviations: CSL, Consortium on Safe Labor; HIV, human immunodeficiency virus; IMPAACT P1025, International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025.
Deliveries to women with HIV were nearly 3 times more likely to have 2 or more chronic comorbid conditions compared with deliveries to women without HIV (10% vs 3%; adjusted odds ratio [AOR]: 2.96; 95% confidence interval [CI]: 2.58–3.41) (Table 2). Similarly, deliveries to women with HIV were more likely to have any chronic comorbid condition (33% vs 20%; AOR: 1.89; 95% CI: 1.73–2.06). Deliveries to women with HIV had the same frequency of obstetrical complications compared with deliveries to women without HIV, with a slightly lower adjusted odds (17% for both; AOR: .84; 95% CI: .75–.94).
Table 2.
Association of Chronic Comorbid Conditions and Obstetrical Complications With Human Immunodeficiency Virus in Pregnancy
| Unadjusted OR (95% CI) | AOR (95% CI)a | |
|---|---|---|
| Primary outcomes | ||
| ≥2 Chronic comorbid conditions | 3.48 (3.07, 3.95) | 2.96 (2.58, 3.41) |
| Obstetric complication | .95 (.86, 1.05) | .84 (.75, .94) |
| Secondary outcomes | ||
| By comorbid condition disease system | ||
| Psychiatric | 4.67 (4.21, 5.18) | 5.13 (4.53, 5.80) |
| Pulmonary | 1.69 (1.51, 1.89) | 1.66 (1.47, 1.87) |
| Cardiovascular | 1.98 (1.74, 2.26) | 1.27 (1.09, 1.47) |
| Endocrine | .79 (.65, .96) | 1.02 (.84, 1.25) |
| Hematologic | 41.24 (31.02, 54.83) | 36.87 (25.80, 52.67) |
| Neurological | 2.34 (1.74, 3.14) | 3.24 (2.40, 4.36) |
| Other diseaseb | .32 (.20, .51) | .26 (.16, .41) |
| By specific obstetric/infant complication | ||
| Pre-eclampsia | .93 (.79, 1.09) | .80 (.67, .95) |
| Gestational diabetes | 1.05 (.87, 1.26) | .70 (.57, .87) |
| Very preterm birth <34 weeks | .82 (.67, 1.00) | .76 (.62, .94) |
| Gestational hypertension | .80 (.63, 1.03) | .90 (.69, 1.17) |
| Fetal growth restriction | 1.05 (.76, 1.47) | 1.45 (1.04, 2.01) |
| Other complicationc | 1.59 (1.21, 2.10) | 1.22 (.91, 1.63) |
| Other secondary outcomes | ||
| Chronic comorbid condition (any) | 1.95 (1.80, 2.11) | 1.89 (1.73, 2.06) |
| Preterm birth <37 weeks | 1.45 (1.31, 1.60) | 1.31 (1.17, 1.47) |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; CSL, Consortium on Safe Labor; HIV, human immunodeficiency virus; IMPAACT P1025, International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025; OR, odds ratio.
aAORs and 95% CIs are the odds of having the given outcome among women with HIV in IMPAACT P1025 compared with women without HIV in CSL (ie, reference group). Generalized logistic regression with within-participant random effect to account for women with multiple deliveries during the study time period was used. Models adjusted for the following covariates: maternal age at delivery (continuous), maternal race/ethnicity (Black, Hispanic, White), parity (0, ≥1), US region (Northeast, West, Midwest, and South including Puerto Rico), and delivery year (continuous). Obstetrical complication model additionally adjusted for multiple gestation (yes or no) and history of preterm birth (yes or no).
bOther disease includes renal disease and gastrointestinal disease.
cOther complication includes placenta previa and fetal demise.
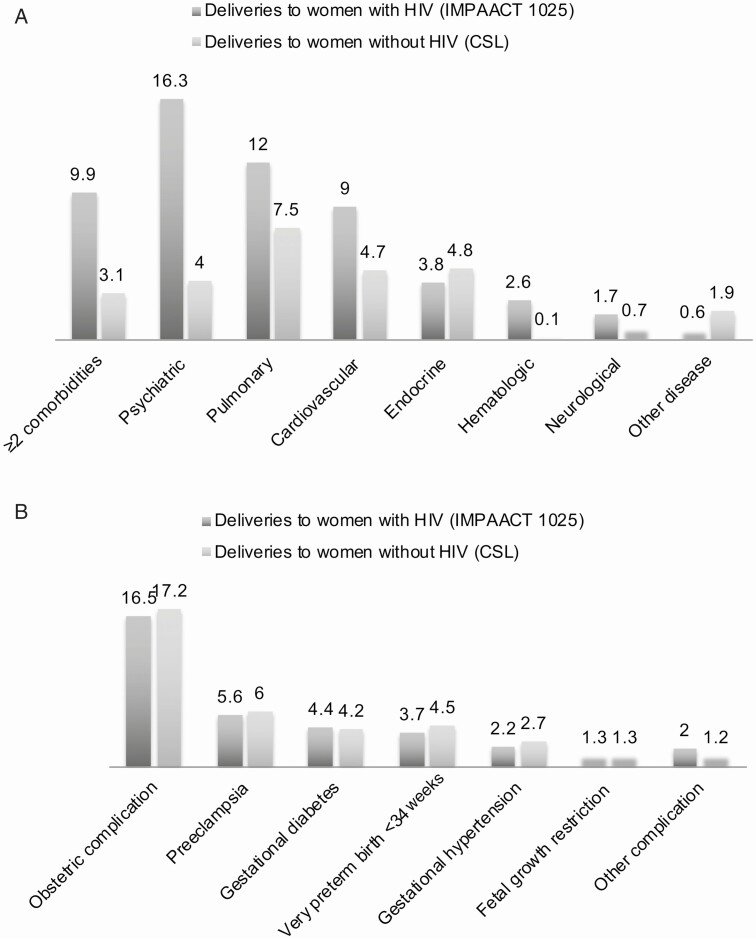
Deliveries to women with HIV were more likely to be complicated by a chronic comorbidity of most disease systems compared with those without HIV, including psychiatric (16% vs 4%), pulmonary (12% vs 8%), cardiovascular (9% vs 5%), hematologic (3% vs 0%), and neurologic (2% vs 1%), but not endocrine, conditions (Figure 2A). The frequency of obstetrical complications was similar between both groups (Figure 2B), but in secondary analyses, preterm birth at fewer than 37 weeks gestation was higher (18% vs 13%) in deliveries with HIV (Supplementary Table 2).
Figure 2.
Frequency (%) of chronic comorbid conditions and obstetric complications among deliveries to women with and without HIV infection. A, Chronic comorbid conditions (%). B, Obstetrical/infant complications (%). The results are presented as frequencies (n and %) in Supplementary Table 2. Abbreviations: CSL, Consortium on Safe Labor; HIV, human immunodeficiency virus; IMPAACT P1025, International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025.
Among deliveries to women with HIV, later entry to prenatal care (≥14 weeks) and starting ART in the earlier time period (2002–2008) were associated with a lower likelihood of 2 or more chronic comorbidities (Table 3). A similar pattern was noted for both later entry to prenatal care and starting ART in the earlier time period for obstetrical complications. A detectable viral load at delivery (≥400 copies/mL) was associated with a higher likelihood of obstetrical complications, but not chronic comorbidity.
Table 3.
Association of Entry to Prenatal Care, Viral Load at Delivery, and Antiretroviral Therapy Guideline Period and 2 or More Chronic Comorbid Conditions and Obstetrical Complications Among Deliveries to Women With Human Immunodeficiency Virus Infection
| ≥2 Chronic Comorbid Conditions (Row Percentage) | Obstetrical Complication (Row Percentage) | |||||
|---|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | AOR (95% CI)a | Yes, n (%) | No, n (%) | AOR (95% CI)a,b | |
| Entry into prenatal care | ||||||
| ≥14 weeks | 75 (8) | 910 (92) | .65 (.50, .86) | 139 (14) | 846 (86) | .76 (.61, .94) |
| <14 weeks | 210 (11) | 1664 (89) | 1.00 | 330 (18) | 1544 (82) | 1.00 |
| Viral load at delivery | ||||||
| ≥400 copies/mL | 50 (11) | 388 (89) | 1.07 (.83, 1.37) | 99 (23) | 339 (77) | 1.32 (1.07, 1.62) |
| <400 copies/mL | 234 (10) | 2150 (90) | 1.00 | 368 (15) | 2016 (85) | 1.00 |
| ART guideline period | ||||||
| 2002–2008 | 124 (8) | 1393 (92) | .72 (.57, .92) | 233 (15) | 1284 (85) | .76 (.63, .93) |
| 2009–2013 | 161 (12) | 1190 (88) | 1.00 | 239 (18) | 1112 (82) | 1.00 |
Abbreviations: AOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; CSL, Consortium on Safe Labor; HIV, human immunodeficiency virus; IMPAACT P1025, International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1025.
aAORs and 95% CIs are the odds of having the given outcome among women with HIV in IMPAACT P1025 compared with women without HIV in CSL (ie, reference group). Generalized logistic regression with within-participant random effect to account for women with multiple delivering during the study time period was used. Models adjusted for the following covariates: maternal age at delivery (continuous), maternal race/ethnicity (Black, Hispanic, White), parity (0, ≥1), US region (Northeast, West, Midwest, and South including Puerto Rico), and delivery year (continuous).
bObstetrical complication model additionally adjusted for multiple gestation (yes or no) and history of preterm birth (yes or no).
In sensitivity analysis, among deliveries in the CSL after including deliveries with HIV (albeit with unknown ART exposure) compared with those without HIV, similar to the primary analysis above, deliveries to women with HIV were associated with increased odds of 2 or more chronic comorbid conditions (6% vs 3%; AOR: 1.82; 95% CI: 1.43–2.69) but not a significantly increased odds of obstetrical complications (21% vs 18%; AOR: 1.18; 95% CI: 1.00–1.40). Next, the frequency of 2 or more chronic comorbid conditions was higher in 2007–2013 compared with 2002–2006 (11% vs 8%; P = .01) among deliveries to women with HIV in IMPAACT P1025, which held for psychiatric illness (18% vs 14%; P = .01) but not other chronic comorbid conditions and obstetrical complications (Supplementary Table 3). In comparison to CSL during the same time period, the frequency of 2 or more chronic comorbid conditions between 2002 and 2006 with HIV in IMPAACT P1025 was higher than without HIV in CSL (8% vs 3%; P < .001). Finally, after excluding 338 women with HIV (12%) receiving a regimen of 3 or more NRTIs, the primary analysis held for 2 or more chronic comorbid conditions (AOR: 2.94; 95% CI: 2.54–3.42) and obstetrical complications (AOR: .87; 95% CI: .78–.98).
DISCUSSION
We found that chronic comorbid conditions are more frequent among US pregnant women with HIV, but not most obstetrical complications, compared with their counterparts without HIV. These findings highlight that, despite increasingly effective ART that has transformed HIV into a chronic disease itself, pregnant women with HIV and receiving ART continue to require high-risk interdisciplinary prenatal care, with an emphasis on management of multiple chronic comorbidities.
Our findings during pregnancy are consistent with emerging data suggesting that individuals with HIV and receiving ART are at high risk of chronic disease, with less comorbidity-free life expectancy compared with their counterparts without HIV [3]. Addressing comorbidities in the setting of HIV with ART is multifactorial and requires addressing chronic immune activation, antiretroviral adverse effects, coinfection, and healthcare disparities [40]. Despite more effective therapy over time, we found that later ART time period was associated with an increased risk of both comorbidities and obstetrical complications, which may be related to rising maternal age and obesity among pregnant women with HIV [41, 42]. A prior analysis using the National Inpatient Sample from 2004 to 2011 showed that, while the number of hospitalizations and deliveries for pregnant women with HIV remained stable, this population was at higher risk of several comorbid conditions, as well as adverse pregnancy/infant outcomes, resulting in more costly and longer hospitalizations [13, 22]. This was consistent with an earlier analysis prior to more effective ART from 1994 to 2003 [21]. Taken together, we recommend continued emphasis on identifying and treating chronic comorbid conditions among pregnant women with HIV. Access to effective ART alone may not be sufficient to fully address their health needs.
In the current study, a striking finding was the high frequency of psychiatric illness among women with HIV enrolled in care and receiving ART. There is an increasing recognition of the high burden of mental health issues among pregnant women with HIV [43] and that depression is associated with nonadherence to ART [44]. Access to ART may be associated with improved mental health due to better physical health, regular access to healthcare services, reduced neurotoxic effects of HIV, and less HIV-related stigma [45]. Furthermore, we also found that pregnant women with HIV were at higher risk of cardiovascular and pulmonary disease but not endocrine disorders. HIV disease progression may result in metabolic complications, renal toxicity, hepatotoxicity, and osteoporosis [40]. Additionally, prolonged use of certain antiretroviral regimens may contribute to an increased risk of cardiovascular disease [1]. Together, individuals with HIV and receiving ART may develop chronic comorbidities at a younger age compared with those without HIV [2].
We found that women living with HIV were not at higher risk of obstetrical complications. In secondary analyses, they were at higher risk of preterm birth at less than 37 weeks’, but not at less than 34 weeks. Prior data have suggested that women with HIV are at higher risk of hypertensive disorders of pregnancy [46], as well as gestational diabetes, regardless of exposure to ART [25]. Growing evidence primarily from resource-limited settings has suggested that certain antiretroviral regimens, particularly those with a protease inhibitor to which 80% of women in this study were exposed, are associated with a higher risk of later preterm birth (ie, <37 weeks) [47, 48].
Women with HIV who enrolled in prenatal care earlier were more likely to have chronic comorbidities and obstetrical complications, and virologic suppression was associated with fewer obstetrical complications. This may be an encouraging finding, suggesting those at higher risk of pregnancy complications sought prenatal care sooner. Because most pregnancies to women with HIV are unplanned [49], improving disease management for chronic comorbidities earlier in pregnancy, or even before, may improve outcomes. However, less than half of postpartum women engage in HIV care beyond the first 3 months following delivery [50]. Women with HIV may be less able to access needed healthcare services due to lower income, inadequate insurance, minority racial status, unemployment, and lower health literacy, all of which are tied to worse health outcomes [41].
This study has the following limitations. Patterns of chronic comorbidities may have changed since the time periods of the 2 assessed cohorts. These results may not hold for pregnant women with HIV who are not on ART at delivery, which is less than 10% in the United States [14, 15]. Current ART guidelines in pregnancy emphasize the use of newer classes of ART, including integrase inhibitors. Additionally, obstetrical care and the management of chronic comorbidities have continued to change. Over time, pregnant women are older, have a higher body mass index (BMI), and report more mental illness, regardless of HIV infection status. Increasing adoption of electronic health records may have increased documentation, resulting in more reporting and creating an impression of greater comorbidity prevalence. However, we did not identify an increase in study outcomes over time. We did not have data on some comorbid conditions known to be higher among individuals with HIV, including chronic kidney disease and substance-use disorders. In addition, important postpartum outcomes, including cardiomyopathy and postpartum depression, were likely not captured. We used 2 studies with varying study protocols for data abstraction, and it is possible that varying case definitions and data-abstraction methods could have introduced information bias. We conducted a sensitivity analysis limited to the CSL study including deliveries to women with HIV, albeit with unknown treatment status, and confirmed our primary analysis. Although we adjusted for many confounding variables, we could not adjust for some important variables, including socioeconomic status and BMI. We categorized comorbid conditions by disease system, consistent with recent analyses, but this approach does not account for disease severity and may group disparate conditions together [1–3]. There was nonoverlapping time periods between both studies, which we addressed with additional sensitivity analyses restricted to concurrent time periods. Sociodemographic characteristics were different between both studies, most notably patient race, with 58% of women with HIV being Black. While we adjusted for race, the risk of comorbidities and obstetrical complications could be affected by racial disparities [16]. Finally, we used 2 studies primarily conducted at tertiary care centers with expertise in HIV and high-risk pregnancies.
Strengths of this study include the comparison of pregnant women from both a cohort of women with HIV and without, unlike prior studies [12, 41], and inclusion of a generally homogenous population of women with HIV on protease inhibitor–containing ART.
In conclusion, we found that chronic comorbidities are more frequent among pregnant US women with HIV and receiving ART, but not obstetrical complications, albeit secondarily higher odds of preterm birth (<37 weeks), compared with women without HIV. These findings highlight the evolving, but continued importance of high-risk interdisciplinary pregnancy care for women with HIV with attention to the management of chronic comorbidities.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. Intramural investigators designed the study and data were collected by clinical site investigators. The corresponding author has full access to the data and final responsibility for preparation and submission of the paper for publication.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases (NIAID) cooperative agreement 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and grant number 1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the NIAID and the National Institute of Child Health and Human Development (NICHD) (grant number U01 AI41110, U01 AI068616, N01-DK-9-001/HHSN267200800001C) International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the NIAID (grant number U01 AI068632); the Eunice Kennedy Shriver (NICHD; and the National Institute of Mental Health (grant number AI068632). The Consortium on Safe Labor (CSL) was funded by the Intramural Research Program of the NICHD, through contract number HHSN267200603425C. Dr. Venkatesh was supported by the Care Innovation and Community Improvement Program at The Ohio State University.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
IMPAACT P1025 Team. Gwendolyn B. Scott, MD, University of Miami School of Medicine, Miami, FL; Elizabeth Smith, MD, National Institute of Allergy and Infectious Diseases Division of AIDS, Pediatric Medicine Branch, Bethesda, MD; Heather Watts, MD, National Institute of Child Health and Human Development, Pediatric, Adolescent, and Maternal AIDS(PAMA) Branch, Bethesda, MD; KaSaundra M. Oden, MHS, International Maternal Pediatric Adolescent AIDS Clinical Trials Group, Silver Spring, MD; T. H., Harvard School of Public Health, Boston, MA; Kunjal Patel, MD, Harvard School of Public Health, Boston, MA; Emily A. Barr, CPNP, CNM, MSN, University of Colorado Denver, The Children’s Hospital, Denver, CO; Diane W. Wara, MD, University of California at San Francisco, San Francisco, CA; Sandra K. Burchett, MD, MSc, Harvard Medical School, Boston, MA; Jenny Gutierrez, MD, Bronx-Lebanon Hospital, Bronx, NY; Kathleen Malee, PhD, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Patricia Tanjutco, MD, Washington Hospital Center, Washington, DC; Yvonne Bryson, MD, David Geffen School of Medicine, University of California, Los Angeles, CA; Michael T. Basar, BS, Frontier Science & Technology Research Foundation, Inc, Amherst, NY; Adriane Hernandez, MA, Frontier Science & Technology Research Foundation, Inc, Amherst, NY; Amy Jennings, BS, Frontier Science & Technology Research Foundation, Inc, Amherst, NY; Tim R. Cressey, PhD, BSc, Program for HIV Prevention & Treatment, Chang Mai, Thailand; Jennifer Bryant, MPA, Westat, Rockville, MD.
Participating sites and site personnel include: 5041 Children’s Hospital of Michigan NICHD CRS(Theodore B. Jones, MD; Ernestine Brown, RN; Natalie Woods, RD); 5052 University of Colorado Denver NICHD Clinical Research Site (CRS) (Alisa Katai, MHA; Tara Kennedy, FNP-BC; Kay Kinzie, MSN, FNP-BC; Jenna Wallace, MSW; Clinical and Translational Sciences Institute (CTSI) grant number UL1 TR000154); 5031 San Juan City Hospital PR NICHD CRS(Rodrigo Diaz- Velasco, MD; Midnela Acevedo-Flores, MD, MT; Elvia Pérez-Hernández, BS, MEd, MA, MPH; Antonio Rodriguez-Mimoso, MD); 5048 USC LA NICHD CRS(Françoise Kramer, MD; LaShonda Spencer, MD; James Homans, MD; Andrea Kovacs, MD); 4601 UCSD Maternal, Child, and Adolescent HIV CRS(Andrew Hull, MD; Mary Caffery, RN, MSN; Jean M Manning RN, BSN; Stephen A. Spector, MD); 4101 Columbia IMPAACT CRS; 4201 University of Miami Pediatric Perinatal HIV/AIDS CRS(Charles D. Mitchell, MD; Salih Yasin, MD; Safia Khan, MD); 5083 Rush University Cook County Hospital Chicago NICHD CRS(Mariam Aziz, MD; Latania Logan, MD; Julie Schmidt, MD; Helen Cejtin, MD); 5096 University of Alabama Birmingham NICHD CRS(Marilyn Crain, MPH, MD; Sharan Robbins, BA; Mickey Parks, CRNP; Yvonne Gamble Duke, MA); 6901 Bronx-Lebanon Hospital IMPAACT CRS(Murli Purswani, MD; Stefan Hagmann, MD, MSc; Mary Vachon, LMSW, MPH); 5012 NYU School of Medicine NICHD CRS(William Borkowsky, MD; Maryam Minter, RN; Aditya Kaul, MD; Nagamah Deygoo, MS); 3801 Texas Children’s Hospital CRS(Shelley Buschur, RN, NMV; Kathleen Pitts, CPNP; Chivon McMullen-Jackson, BSN, RN; Theresa Aldape, LMSW); 4001 Chicago Children’s CRS(Donna McGregor, RN); 5009 Children’s Hospital of Boston NICHD CRS(Arlene Buck, RN; Catherine Kneut, RN, CPNP); 5018 USF—Tampa NICHD CRS(Patricia Emmanuel, MD; Karen Bruder, MD; Gail Lewis, RN); 6501 St Jude/The University of Tennessee Health Science Center (UTHSC) CRS(Katherine Knapp, MD; Edwin Thorpe, MD; Nina Sublette, FNP, PhD; Pam Finnie, MSN); 2802 NJ Medical School CRS(Charmane Calilap-Bernardo, RN; Linda Bettica, RN); 3601 UCLA-Los Angeles/Brazil AIDS Consortium(LABAC) CRS(Jaime G. Deville, MD; Karin Nielsen-Saines, MD; Nicole Falgout, RN; Michele Carter, RN); 4005 Mt Sinai Hospital Med Center, Women’s & Children’s HIV Program(Brenda Wolfe, APN; Molly Hartrich, MPH); 5017 Seattle Children’s Hospital CRS; 5023 Washington Hospital Center NICHD CRS(Steven Zeichner, MD, PhD; Sara R. Parker, MD; Vanessa Emmanuel, BA); 5028 University of Illinois College of Medicine at Chicago, Department of Pediatrics(Kenneth Rich, MD; Karen Hayani, MD; Julia Camacho, RN); 5051 University of Florida College of Medicine, Jacksonville(Mobeen Rathore, MD; Ayesha Mirza, MD; Nizar Maraqa, MD; Kathleen Thoma, MA, CCRP); 5094 University of Maryland Baltimore NICHD CRS(Douglas Watson, MD; Corinda Hilyard); 6601 University of Puerto Rico Pediatric HIV/ AIDS Research Program CRS(Irma L. Febo, MD; Vivian Tamayo, MD; Ruth Santos, RN, MPH; Maritza Cruz- Rodriguez); 5003 Metropolitan Hospital NICHD CRS; 5013 Jacobi Medical Center Bronx NICHD CRS(Susan Gross, MD; Michael Moore, MD; Carmen Caines, RN); 5038 Yale University School of Medicine; 5045 Harbor UCLA Medical Center NICHD CRS(Margaret Keller, MD; Spring Wettgen, RN, PNP; Judy Hayes, RN; Yolanda Gonzalez, RN); 5095 Tulane University New Orleans NICHD CRS(Yvette Luster, RN; Robert Maupin, MD; Chi Dola, MD; Margarita Silio, MD); 6701 The Children’s Hospital of Philadelphia IMPAACT CRS(Steven D. Douglas, MD; Richard M. Rutstein, MD; Carol A. Vincent, CRNP, MSN).
Institutions involved in the Consortium on Safe Labor include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, UT; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville, MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, TX.
Presented at: the Society for Maternal Fetal Medicine 41st Annual Pregnancy Meeting, virtual, 26 January 2021. Poster presentation #538.
References
- 1.Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection—a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 2.Lerner A, Eisinger RW, Fauci AS. Comorbidities in persons with HIV: the lingering challenge. J Am Med Assoc JAMA 2020; 323:19–20. [DOI] [PubMed] [Google Scholar]
- 3.Marcus J, Leyden WA, Alexeeff SE, et al. . Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. J Am Med Assoc Network Open 2020; 3:e207964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallant J, Hsue P, Budd D, Meyer N. Healthcare utilization and direct costs of non-infectious comorbidities in HIV-infected patients in the USA. Curr Med Res Opin 2018; 34:13–23. [DOI] [PubMed] [Google Scholar]
- 5.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol 2012; 206:134, e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrick SW, Cooper WO, Davis MM. Prescribing opioids and psychotropic drugs in pregnancy. BMJ 2017; 358:j3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J 2015; 19:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995–2006. Br J Obstet Gyneacol 2011; 118:345–52. [DOI] [PubMed] [Google Scholar]
- 9.Ellington S, Flowers L, Legardy-Williams JK, Jamieson DJ, Kourtis AP. Recent trends in hepatic diseases during pregnancy in the United States, 2002–2010. Am J Obstet Gynecol 2015; 212:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mhyre JM, Bateman BT, Leffert LR. Influence of patient comorbidities on the risk of near-miss maternal morbidity or mortality. Anesthesiology 2011; 115:963–72. [DOI] [PubMed] [Google Scholar]
- 11.Admon LK, Winkelman TNA, Heisler M, Dalton VK. Obstetric outcomes and delivery-related health care utilization and costs among pregnant women with multiple chronic conditions. Prev Chronic Dis 2018; 15:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend CL, de Ruiter A, Peters H, Nelson-Piercy C, Tookey P, Thorne C. Pregnancies in older women living with HIV in the UK and Ireland. HIV Med 2017; 18:507–12. [DOI] [PubMed] [Google Scholar]
- 13.Bansil P, Jamieson DJ, Posner SF, Kourtis AP. Hospitalizations of pregnant HIV-infected women in the United States in the era of highly active antiretroviral therapy (HAART). J Womens Health (Larchmt) 2007; 16:159–62. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. HIV among pregnant women, infants, and children. Atlanta, GA: Centers for Disease Control and Prevention, 2016. [Google Scholar]
- 15.Taylor A, Nesheim SR, Zhang X, et al. . Estimated perinatal HIV infection among infants born in the United States, 2002–2013. JAMA Pediatrics 2017; 171:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz IT, Leister E, Kacanek D, et al. . Factors associated with lack of viral suppression at delivery among highly active antiretroviral therapy-naive women with HIV: a cohort study. Ann Intern Med 2015; 162:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powis K, Huo Y, Williams PL, et al. ; Pediatric HIV/AIDS Cohort Study (PHACS) . Antiretroviral prescribing practices among pregnant women living with HIV in the United States, 2008–2017. JAMA Network Open 2019; 2:e1917669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson BL, Cu-Uvin S. Pregnancy and optimal care of HIV-infected patients. Clin Infect Dis 2009; 48:449–55. [DOI] [PubMed] [Google Scholar]
- 19.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1- infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed 26 October 2020.
- 20.Venkatesh KK, Morrison L, Livingston EG, et al. . Changing patterns and factors associated with mode of delivery among pregnant women with human immunodeficiency virus infection in the United States. Obstet Gynecol 2018; 131:879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kourtis AP, Bansil P, McPheeters M, Meikle SF, Posner SF, Jamieson DJ. Hospitalizations of pregnant HIV-infected women in the USA prior to and during the era of HAART, 1994-2003. AIDS 2006; 20:1823–31. [DOI] [PubMed] [Google Scholar]
- 22.Ewing A, Datwani HM, Flowers LM, Ellington SR, Jamieson DJ, Kourtis AP. Trends in hospitalizations of pregnant HIV-infected women in the United States: 2004 through 2011. Am J Obstet Gynecol 2016; 215:499. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesh KK, Farhad M, Fenton T, et al. . Association between HIV antiretroviral therapy and preterm birth based on antenatal ultrasound gestational age determination: a comparative analysis. AIDS 2019; 33:2403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowler MG, Qin M, Fiscus SA, et al. ; IMPAACT 1077BF/1077FF PROMISE Study Team . Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitter A, Stücker AU, Linde R, et al. . Pregnancy complications in HIV-positive women: 11-year data from the Frankfurt HIV cohort. HIV Med 2014; 15:525–36. [DOI] [PubMed] [Google Scholar]
- 26.Tshivuila-Matala COO, Honeyman S, Nesbitt C, Kirtley S, Kennedy SH, Hemelaar J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS 2020; 34:1643–56. [DOI] [PubMed] [Google Scholar]
- 27.Buttorff C, Ruder T, Bauman M.. Multiple chronic conditions in the United States. Santa Monica, CA: RAND Corporation, 2017. [Google Scholar]
- 28.Roberts ER, Green D, Kadam UT. Chronic condition comorbidity and multidrug therapy in general practice populations: a cross-sectional linkage study. BMJ Open 2014; 4:e005429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livingston EG, Huo Y, Patel K, Tuomala RE, Scott GB, Stek A; P1025 Team of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group . Complications and route of delivery in a large cohort study of HIV-1-infected women—IMPAACT P1025. J Acquir Immune Defic Syndr 2016; 73:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Troendle J, Reddy UM, et al. ; Consortium on Safe Labor . Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 2010; 203:326, e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatesh KK, Glover AV, Vladutiu CJ, Stamilio DM. Association of chorioamnionitis and its duration with adverse maternal outcomes by mode of delivery: a cohort study. BJOG 2019; 126:719–27. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Multimorbidity.2016. Available at: http://www.who.int/iris/handle/10665/252275. Accessed 26 October 2020.
- 33.National Institute for Health and Care Excellence. Multimorbidity: clinical assessment and management. Available at: https://www.nice.org.uk/guidance/ng56. Accessed 26 October 2020.
- 34.Prins JR, Holvast F, van’t Hooft J, et al. . Development of a core outcome set for immunomodulation in pregnancy (COSIMPREG): a protocol for a systematic review and Delphi study. BMJ Open 2018; 8:e021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubert CE, Schnipper JL, Roumet M, et al. . Best definitions of multimorbidity to identify patients with high health care resource utilization. Mayo Clin Proc Innov Qual Outcomes 2020; 4:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 10:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesh KK, Strauss RA, Westreich DJ, Thorp JM, Stamilio DM, Grantz KL. Adverse maternal and neonatal outcomes among women with preeclampsia with severe features <34 weeks gestation with versus without comorbidity. Pregnancy Hypertens 2020; 20:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. International statistical classification of diseases and related health problems. 10th revision. Geneva, Switzerland: World Health Organization, 2007.
- 39.Hutcheon JA, Moskosky S, Ananth CV, et al. . Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol 2019; 33:O15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guaraldi G, Prakash M, Moecklinghoff C, Stellbrink HJ. Morbidity in older HIV-infected patients: impact of long-term antiretroviral use. AIDS Rev 2014; 16:75–89. [PubMed] [Google Scholar]
- 41.Cunningham WE, Hays RD, Duan N, et al. . The effect of socioeconomic status on the survival of people receiving care for HIV infection in the United States. J Health Care Poor Underserved 2005; 16:655–76. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesh K, Edmonds A, Westreich D, et al. . Associations between HIV, antiretroviral therapy, and preterm birth in the Women’s Interagency HIV Study, 1994–2018. Presented at: Infectious Diseases for Obstetrics and Gynecology Annual Meeting; Virtual meeting, August 14, 2020.
- 43.Kaida A, Matthews LT, Ashaba S, et al. . Depression during pregnancy and the postpartum among HIV-infected women on antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr 2014; 67(Suppl 4):S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammassari A, Antinori A, Aloisi MS, et al. . Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics 2004; 45:394–402. [DOI] [PubMed] [Google Scholar]
- 45.Tsai AC, Weiser SD, Steward WT, et al. . Evidence for the reliability and validity of the internalized AIDS-related stigma scale in rural Uganda. AIDS Behav 2013; 17:427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansone M, Sarno L, Saccone G, et al. . Risk of preeclampsia in human immunodeficiency virus-infected pregnant women. Obstet Gynecol 2016; 127:1027–32. [DOI] [PubMed] [Google Scholar]
- 47.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21:607–15. [DOI] [PubMed] [Google Scholar]
- 48.Powis KM, Kitch D, Ogwu A, et al. . Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis 2011; 204:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutton MY, Patel R, Frazier EL. Unplanned pregnancies among HIV-infected women in care-United States. J Acquir Immune Defic Syndr 2014; 65:350–8. [DOI] [PubMed] [Google Scholar]
- 50.Adams JW, Brady KA, Michael YL, Yehia BR, Momplaisir FM. Postpartum engagement in HIV care: an important predictor of long-term retention in care and viral suppression. Clin Infect Dis 2015; 61:1880–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.