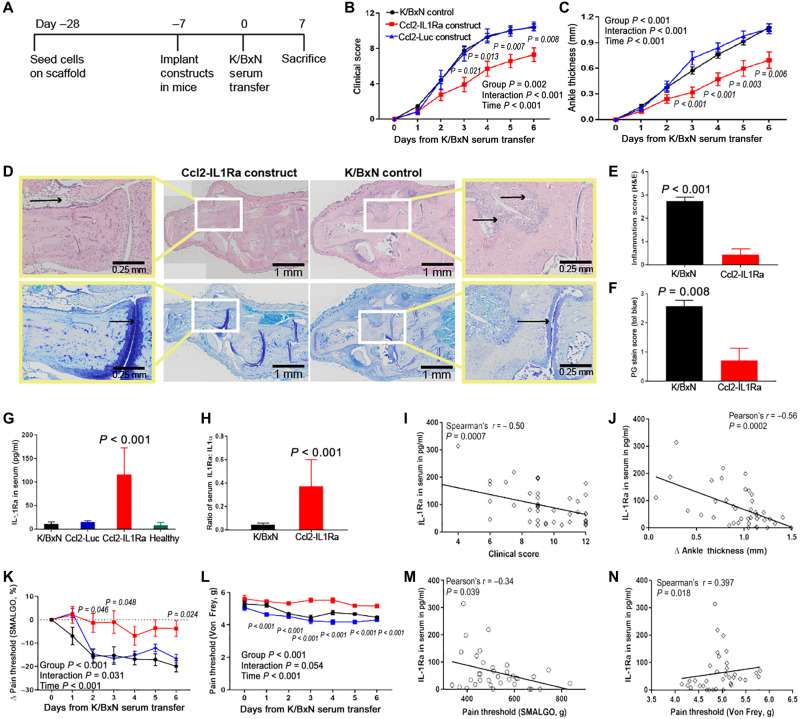
Fig. 5. Ccl2-IL1Ra bioartificial implants mitigate disease severity, inflammation, and pain in mice with K/BxN STA.
(A to C) Ccl2-IL1Ra implants mitigated disease severity as measured by clinical scores and ankle thickness, in contrast to control groups (n = 20, 12, and 8, respectively). Two-way repeated measure ANOVA with Geisser-Greenhouse corrections was calculated, and main effects are displayed in each graph. Adjusted P values for Ccl2-IL1Ra versus Ccl2-Luc scaffolds are shown in italics near each time point. (D to F) At sacrifice, these implants reduced inflammation [(E), n = 8 and 9, left to right] and maintained cartilage integrity [(F), n = 7 per group]. Zoomed images are shown in the yellow boxes, and black arrows illustrate differential inflammatory cell infiltration and proteoglycan staining between treated and untreated groups. (G to J) IL-1Ra levels and ratio of IL-1:IL-1Ra in serum were increased in mice with Ccl2-IL1Ra implants compared to all other groups, and IL-1Ra concentration in serum had negative relations with clinical scores and ankle thickness [(G), n = 10, 6, 12, and 8, left to right; (H), n = 10 per group]. (K to N) Mice that received Ccl2-IL1Ra implants maintained their pain thresholds, indicated by algometry and electronic Von Frey pain tests in contrast to all other groups and significantly related with IL-1Ra concentration in serum (n = 20, 12, and 8, respectively). All values represent means ± SEM. (B, C, K, and L) Two-way repeated measures ANOVA. (E and F) One-sided Mann-Whitney U test. (G) One-way ANOVA. (H) Student’s t test.