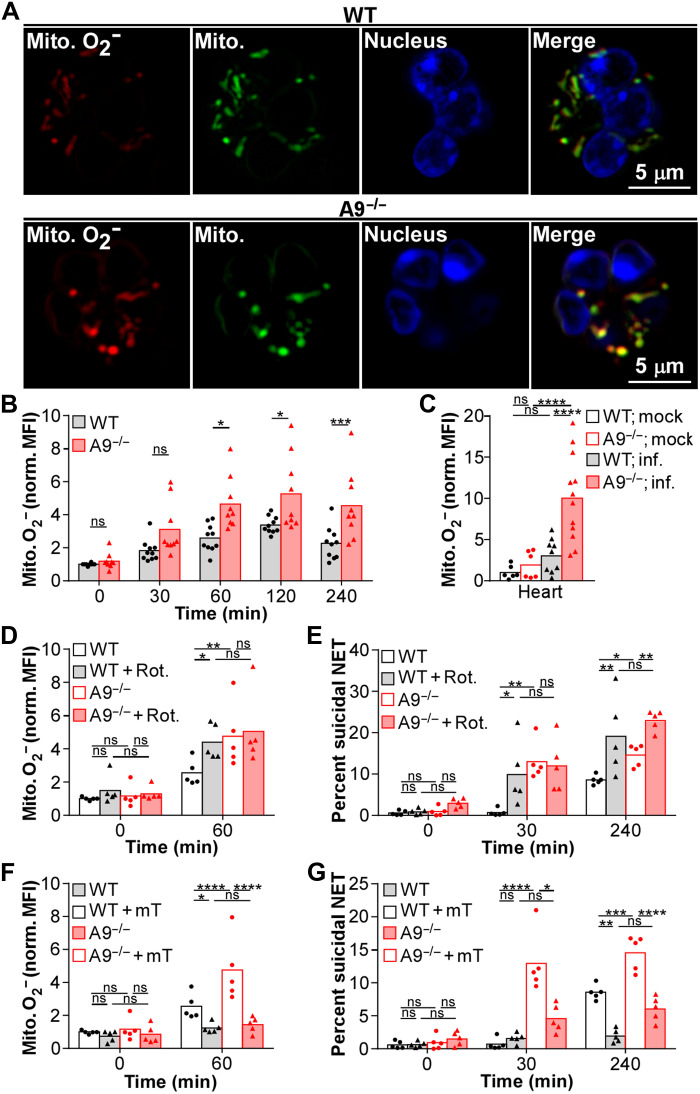
Fig. 3. Increased mitochondrial O2− heightens suicidal NETosis in A9−/− neutrophils responding to S. aureus.
(A and B) Neutrophils were cultured with S. aureus (MOI = 10). (A) Neutrophils were stimulated with S. aureus for 1 hour, and representative images of mitochondrial (Mito.) O2− (red; MitoSOX) and biomass (green; MitoTracker) are provided. Nuclear DNA was stained (blue; Hoechst). (B) Neutrophils (Ly6G+CD11b+) were cultured with S. aureus, and production of mitochondrial O2− was quantified by flow cytometry. MitoSOX MFI was normalized by MitoTracker MFI. Each point represents neutrophils isolated from a single mouse (n = 9). (C) Mice were systemically infected (inf.) with S. aureus (CFU = 2 × 107). At 4 dpi, organs were homogenized and production of mitochondrial O2− in neutrophils was quantified in the heart by flow cytometry. MitoSOX MFI was normalized by MitoTracker MFI. Each point represents a single mouse (mock, n = 6) (WT; inf., n = 9) (A9−/−; inf., n = 12). (D to G) Neutrophils pretreated with (D and E) rotenone (Rot.; 0.5 μM) for 15 min or (F and G) MitoTEMPO (mT; 0.5 μM) for 2 hours were cultured with S. aureus (MOI = 10). Neutrophils were stimulated with S. aureus, and (D and F) the production of mitochondrial O2− and (E and G) the percentage of neutrophils undergoing suicidal NETosis (Dead: extracellular dsDNA+MPO+H3Cit+) were quantified by flow cytometry. (D and F) MitoSOX MFI was normalized by MitoTracker MFI. Each point represents neutrophils isolate from a single mouse (n = 5). Two-way ANOVA with (B) Sidak’s or (C to G) Tukey multiple comparisons test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001).