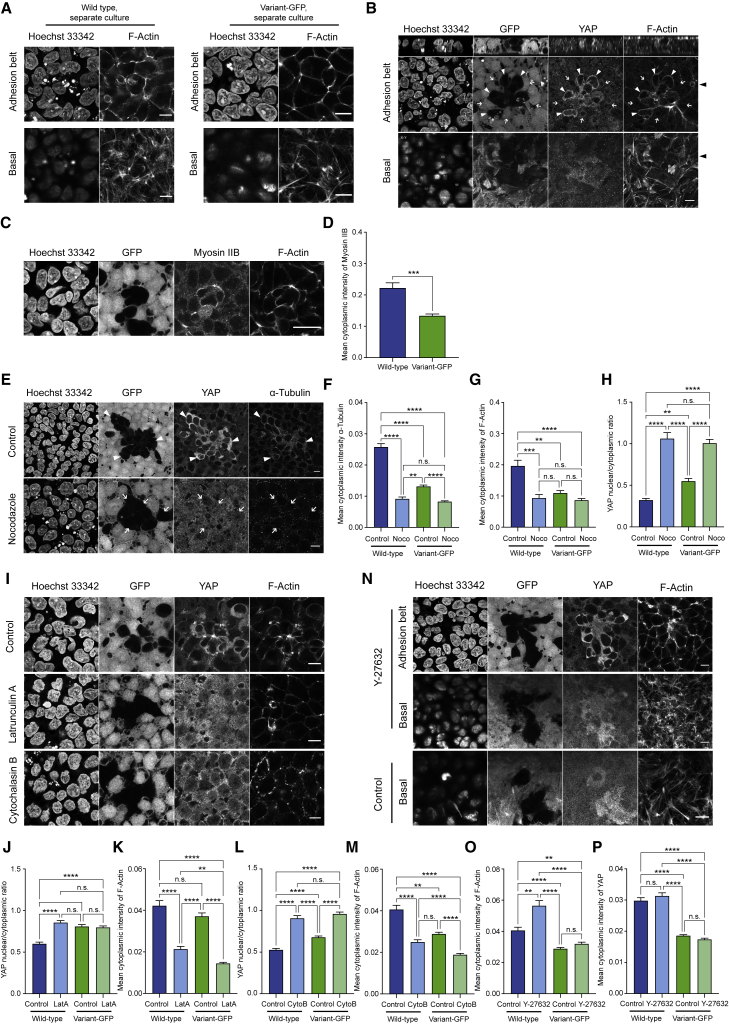
Figure 6.
YAP localization is regulated by adhesion belt actin in hPSCs
(A) F-actin staining at the adhesion belt and basal planes in wild-type (left panels) and variant-GFP (right panels) grown in separate cultures.
(B) F-actin staining at the adhesion belt and basal planes of wild-type and variant-GFP co-cultured cells. Closed arrowheads point to wild-type cells displaying YAP localized within the cytoplasm and having a prominent staining of adhesion belt F-actin. Open arrows point to neighboring variant-GFP cells displaying nuclear localization of YAP and no prominent adhesion belt.
(C) Myosin IIB and F-actin staining within the adhesion belt of co-cultured wild-type and variant-GFP cells.
(D) The mean intensity of myosin IIB in wild-type and variant-GFP cells upon co-culture. ∗∗∗p < 0.001, Kolmogorov-Smirnov test.
(E) Localization of YAP in co-cultured wild-type and variant-GFP cells treated with nocodazole. Closed arrowheads point to wild-type cells displaying YAP localized within the cytoplasm and having prominent staining of α-tubulin. Nocodazole treatment perturbed the microtubule structure and caused diffuse localization of YAP in wild-type cells (open arrows).
(F–H) The mean intensity of α-tubulin (F), F-actin (G), and the nuclear to cytoplasmic ratio of YAP (H), in wild-type and variant-GFP cells ± nocodazole.
(I–M) Disruption of F-actin in the adhesion belt of co-cultured wild-type and variant-GFP cells treated with latrunculin A or cytochalasin B.
(N–P) Treatment of co-cultures with Y-27632.
Data are represented as the mean ± SEM (F–H, J–P); n.s., nonsignificant; ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; one-way ANOVA followed by Kruskal-Wallis multiple comparisons test.
Scale bars: 10 μm.