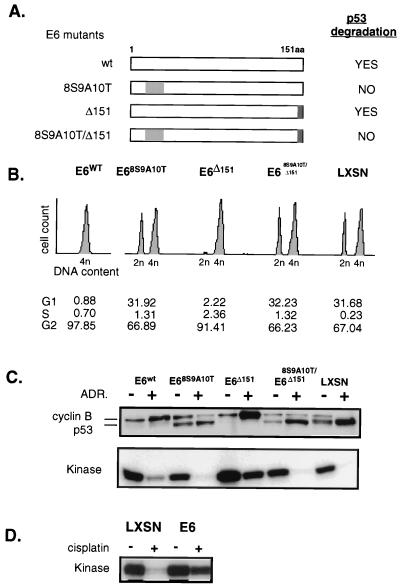
FIG. 2.
Down regulation of cyclin B, CDC2, and kinase activity is a function of p53, rather than E6 or agent of DNA damage used. (A) E6 mutants vary in the ability to degrade p53. E6 is a 151-amino-acid protein, depicted in a linear diagram. Amino acids 8 to 10 are responsible for binding to p53, leading to its degradation. The gray shading of this region indicates E6 mutants with replacement of these amino acids such that p53 binding is disrupted and p53 degradation does not occur. The darker shading indicates deletion of the C-terminal amino acid, 151, which would disrupt E6 binding to hDLG, as described in the text. (B) Asynchronous HFFs transduced with the various mutants were treated with complete medium containing 100 nM ADR for 24 h. Cell cycle profiles were analyzed by flow cytometry. (C) Western blotting and kinase assays were performed with proteins harvested from same cells with and without continuous exposure to ADR for 24 h as for panel B. Cyclin B and kinase down regulation occurs only in the cells capable of DNA damage induced up regulation of p53. (D) The p53-dependent down regulation of kinase activity is not a unique finding in cells DNA damaged with ADR. E6-HFFs and LXSN-HFFs were treated with cisplatin (1 μg/ml) continuously for 24 h. Cyclin B-associated kinase activity assays performed with protein lysates obtained from cells treated or untreated, as indicated, show the LXSN cells with extinguished kinase activity, while the E6 cells maintain high kinase activity.