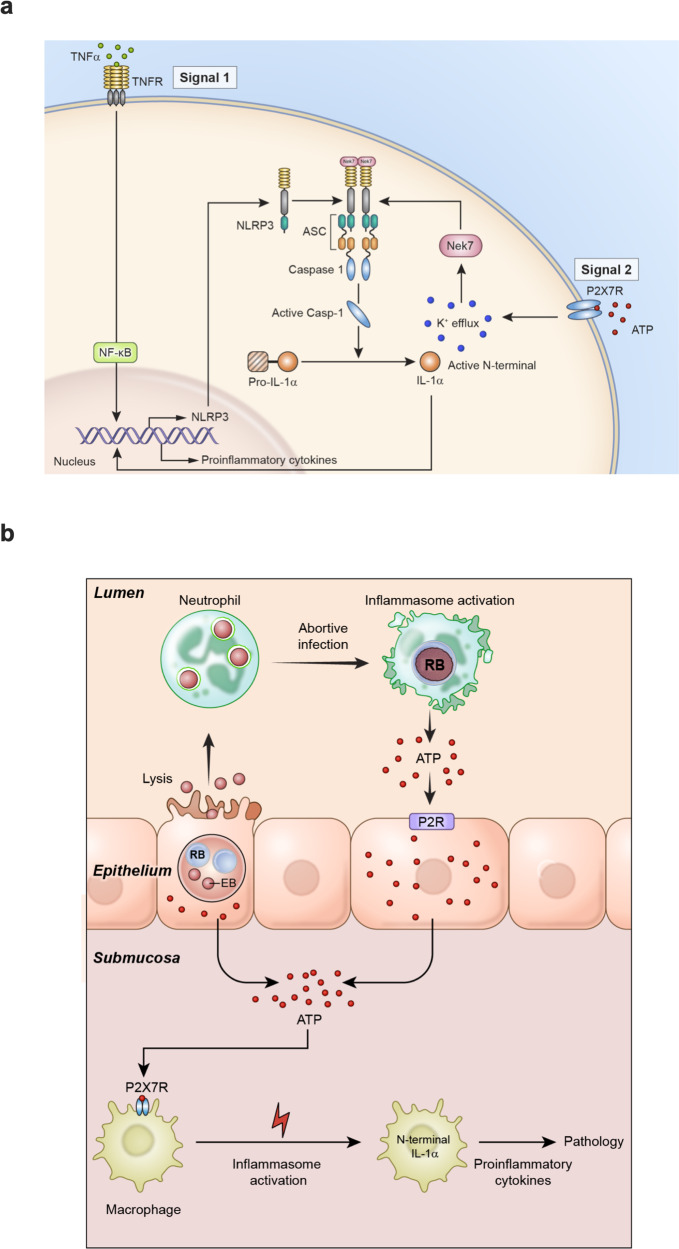
Fig. 7. A model of sterile inflammation generated by chlamydial infection of uterine epithelium.
a NLRP3 inflammasome activation through ATP-P2X7 signaling pathway in phagocytic cells. b ATP released from chlamydiae infected oviduct epithelial cells (Fig. 6a) and luminal neutrophils function (i) directly through penetrating the lamina propria facilitated by matrix metallopeptidase 9 (MMP9) degradation of extracellular matrix molecules that has been shown to amplify the inflammatory response to chlamydial infection56, or (ii) by paracrine/autocrine via P2R cascading purinergic signaling and exacerbation of the macrophage inflammatory response57. Because immunopathology occurs independently of the IL-1R and various PAMPs, we propose the processed N-terminal IL-1α is translocated to the nucleus to transcriptionally regulate pro-inflammatory cytokines which drive immunopathology. Notably, macrophage-associated immunopathology occurred independently of detectable chlamydial infection (Fig. 1i) presenting a model where mucosal infection generated DAMP signaling, which triggers sterile macrophage-associated inflammation in submucosal tissue.