ABSTRACT
Natural killer (NK) cell enteropathy is a newly described disease entity with benign behavior and an indolent clinical course, characterized by the atypical proliferation of NK cells throughout the gastrointestinal tract. The exact etiology is unknown. It closely mimics NK/T-cell lymphoma. We describe this atypical entity in a young adult man presenting with vague upper gastrointestinal symptoms and anemia requiring blood transfusion. The response to budesonide therapy points toward a possible low-grade autoimmune process. Considering the benign behavior and self-limiting course, recognizing this entity is essential to avoid over the investigation and aggressive, inappropriate therapy.
INTRODUCTION
Natural killer (NK) cells, a diverse group of innate lymphoid cells, are subsets of lymphocytes exhibiting cytotoxic function against viruses and tumor cells in lymphoid tissue, spleen, peripheral blood, and extranodal sites, such as gastrointestinal mucosa.1 These cells have the ability to kill tumor cells without previous priming.2 Natural killer cell enteropathy (NKCE) is characterized by an atypical proliferation of CD56 expressing NK cells within the gastrointestinal mucosa.3 The NK cell proliferative diseases have been termed as either NKCE, if it involves any part of the gastrointestinal tract, or lymphomatoid gastropathy, if it is limited to the stomach alone.4 Although NKCE histopathologically mimics aggressive NK/T-cell lymphomas of the gastrointestinal tract, clinically, it follows a benign course.5 Its nonspecific clinical presentation with the limited available literature and lack of established diagnostic criteria often make the diagnosis difficult, leading to unnecessary investigations and more aggressive therapy.6 The index patient has significant symptomatic morbidity with mucocutaneous manifestation and shown a good response to therapy.
CASE REPORT
A 26-year-old man presented with recurrent oro-genital ulceration associated with significant weight loss of 8-year duration. Oral ulcers were recurrent and painful, whereas genital ulcer on glans was painless, indurated, without any discharge or scar on healing, and not concurrent with oral ulceration. After a couple of years of onset of initial symptoms, he experienced postprandial fullness and epigastric discomfort accompanied by intermittent episodes of nonbilious vomiting. He required frequent blood transfusion for falling hemoglobin level. There was no history of joint pain, vision abnormality, altered bowel habits, and B symptoms. The patient was not on any nonsteroidal antiinflammatory drugs or complementary and alternative medicine.
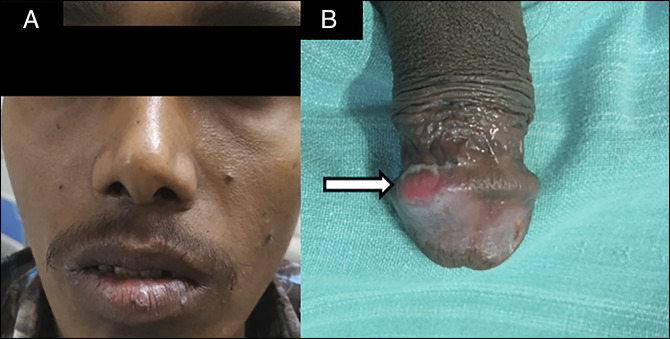
Physical examination revealed mild pallor, painful ulcers on lips and vestibule of the mouth, circumscribed ulcer on the glans penis, and low body mass index (17.5 kg/m2) without any lymphadenopathy and organomegaly. However, unlike in Behcet disease, the ulcers here were painless and nonscarring and were present on the glans penis (uncommon site in the Behcet disease) (Figure 1). Laboratory examination showed a low hemoglobin of 7 g/dL with microcytic hypochromic picture on peripheral smear without any atypical cells with low B12 level 158 ng/mL, low normal serum ferritin 17.2 ng/mL, and normal serum folate levels. Esophagogastroduodenoscopy revealed pangastric nodular mucosa with multiple superficial ulcers and occasional discrete ulcers in the first and second part of the duodenum (Figure 2). Ileocolonoscopy showed multiple small aphthae scattered throughout colon and terminal ileum (Figure 3). Serum gastrin, immunoglobin profile, and anti-tTG antibodies were within the normal range. Bone marrow examination revealed increased megaloblastic maturation. Ocular examination was normal. The pathergy test was negative.
Figure 1.
(A) Ulcers on lips and vestibule of the mouth and (B) circumscribed ulcer on glans penis.
Figure 2.
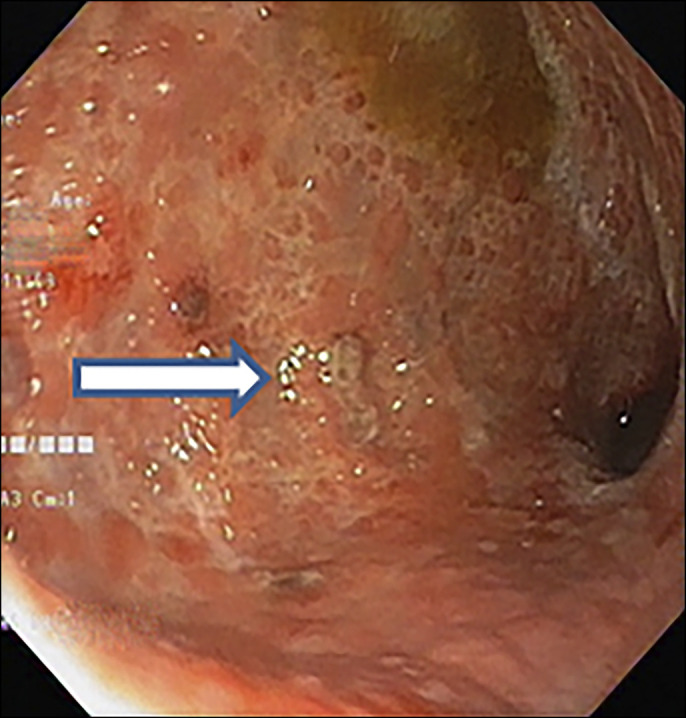
Esophagogastroduodenoscopy revealed pangastric nodular mucosa with multiple superficial ulcers.
Figure 3.
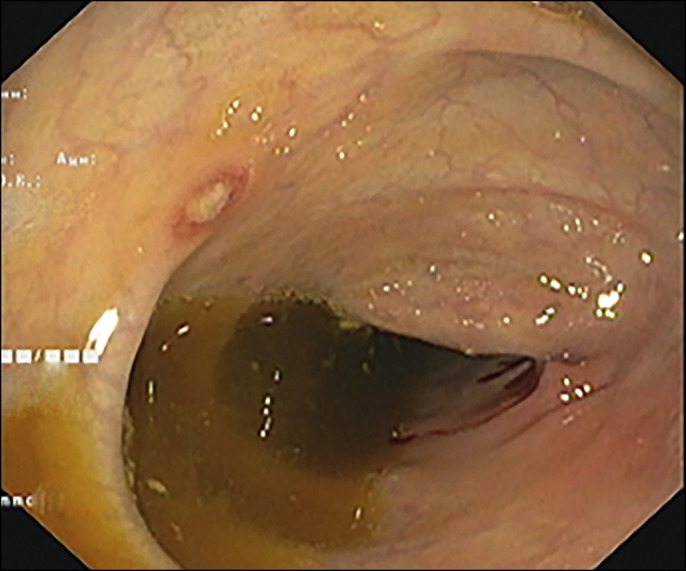
Colonoscopy showing aphthous ulcers in the entire colon.
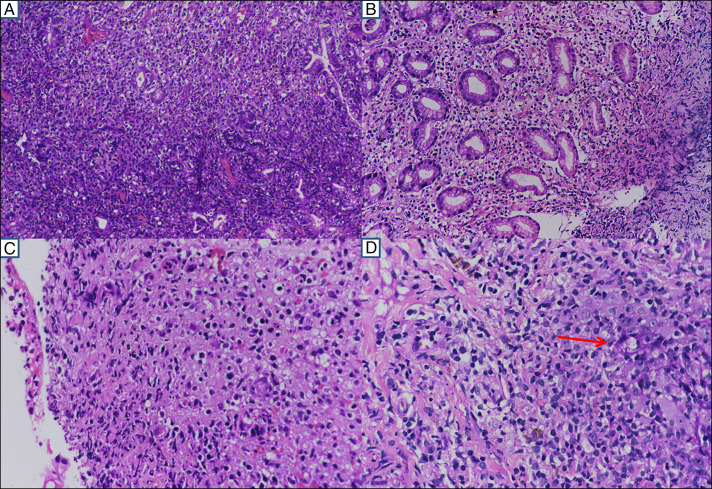
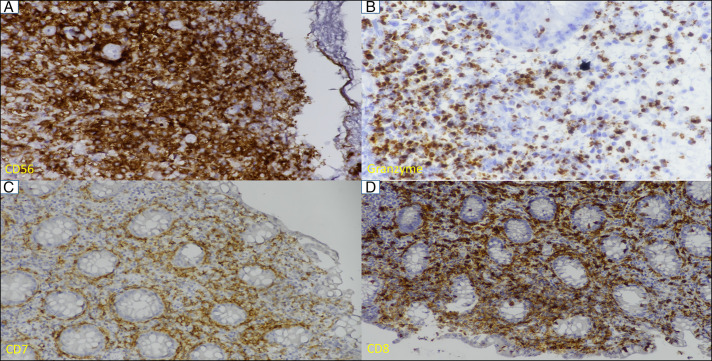
The histopathological findings of mucosal biopsies from the stomach, duodenum, ileum, and colon were similar in nature but variable in intensity. The gastric mucosal lesion mimics mucosa-associated lymphoid tissue lymphoma with mixed cellular infiltrates of lymphocytes, histiocytes, eosinophils, and neutrophils expanding the glands with some lympho-epithelio-trophism (Figure 4). The overlying epithelium was ulcerated. The histiocytoid cells were medium-sized with vacuolated to eosinophilic cytoplasm and centrally placed round to oval to some irregular and indented nuclei, fine chromatin, and some inconspicuous nuclei (Figure 4). No Helicobacter pylori or typical lymphoepithelial lesions were seen. There was no evidence of vasculitis, granuloma, necrosis, or significant mitosis. Punch biopsy from the penile ulcer showed parakeratosis and acanthosis with elongation of rete pegs. The dermal infiltrates are seen infiltrating the rete pegs (red arrow) with similar nuclear and cytoplasmic features (Figure 4). These atypical cells from the gastric antrum, cecum, ascending colon, rectum, and penile skin were strongly positive for CD3, CD7, CD56, and granzyme with a low proliferative index (molecular immunology Boratel-1 1%–5%). Other immune markers were as described (Figure 5). The immune stain for the Epstein-Barr virus was negative. All these findings were consistent with NKCE with extraintestinal involvement.
Figure 4.
(A) Diffuse infiltrate of atypical lymphoid cells displaying the gastric glands (20× magnification), (B) similar infiltrate with vacuolated cytoplasm (20× magnification), (C) ulcerated colonic mucosa with a similar type of infiltrate with slight nuclear irregularity, eosinophilic to vacuolated cytoplasm (40× magnification), and (D) the dermal infiltrates are seen infiltrating the rete pegs (red arrow) with similar nuclear and cytoplasmic features (40× magnification).
Figure 5.
(A) Atypical infiltrates are strong and diffusely positive for CD56 (40× magnification), (B) cytoplasmic granular positivity for granzyme (40× magnification), and (C and D) membranous positivity for CD7 and CD8 (20× magnification).
The patient came to our center with the diagnosis of mucosa-associated lymphoid tissue lymphoma and already has had inadequate treatment for H. pylori. Hence, a course of triple therapy for H. pylori was repeated at our center when he was undergoing further workup. Because we do not have any recommended therapy for NKCE, and considering its similarity in disease distribution and endoscopic mucosal lesions with that of the Crohn's disease, he was initiated with an overlapping regimen of budesonide and azathioprine after the diagnosis of NKCE was established. Budesonide was discontinued after 3 months. He was maintained on 50 mg of azathioprine. At the end of 6 months, he was completely asymptomatic with 3 kg weight gain. The penile ulcer has healed up. He did not require any blood transfusion subsequently.
DISCUSSION
NK cell enteropathy is a disease of the proliferation of atypical NK cells of unknown origin. We report a case of NKCE in a 26-year-old man who was symptomatic for 8 years. The presence of significant weight loss, anorexia, vomiting, and dimorphic anemia, requiring multiple transfusions, indicates extensive pan-gastrointestinal pathology, which was corroborated with endoscopic and mucosal biopsies. The resident NK cells of the gastrointestinal tract are devoid of cytotoxic functions and have a low level of T-cell restricted intracellular antigen-1 and granzyme B. In this patient and other reported series, there was relatively increased expression of granzyme B, indicating acquisition of cytotoxic function, probably as a response to local inflammation, autoimmunity, or an unknown viral infection.
The presence of atypical NK cells in the penile ulcer and in the gastrointestinal tract lesion indicative of a systemic nature of the disease. This extraintestinal manifestation of this entity has further complicated the scenario and would generate more interest in the future. NK/T cell lymphoma is a close mimicker of NKCE. However, the lack of monoclonality and the indolent clinical course of the NKCE help to distinguish it from this aggressive lymphoma. Thus, an accurate diagnosis would avoid inappropriate over-investigation and aggressive therapy.5,6 There were conflicting reports of NKCE's response to anti-H. pylori treatment.3,5,7 The other peculiarity of our case is strong CD8 positivity in all the sites of involvement.8 The presence of symptomatic response to budesonide therapy possibly points toward the fact that this could be secondary to a low-grade autoimmune process.
DISCLOSURES
Author contributions: MK Panigrahi, C. Kumar, HK Nayak, MI Chouhan, and SJ Bhat wrote the article. S. Patra and P. Ayyanar provided the pathology images. SC Samal edited the article and is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Manas Kumar Panigrahi, Email: manaskumarpanigrahi@gmail.com.
Susama Patra, Email: pathol_susama@aiimsbhubaneswar.edu.in.
Chandan Kumar, Email: chandan8867@gmail.com.
Hemanta Kumar Nayak, Email: drhemantnayak@gmail.com.
Pavithra Ayyanar, Email: pavithraayyanar@gmail.com.
Sunil Jee Bhat, Email: sunilbhat123.sb@gmail.com.
Mohd Imran Chouhan, Email: chouhan.imran43@gmail.com.
REFERENCES
- 1.Tagliabue A, Befus AD, Clark DA, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982;155:1785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogenic tumours. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–29. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi K, Yokoyama M, Ishizawa S, et al. Lymphomatoid gastropathy: A distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116:5631–7. [DOI] [PubMed] [Google Scholar]
- 4.Ganapathi KA, Pittaluga S, Odejide OO, Freedman AS, Jaffe ES. Early lymphoid lesions: Conceptual, diagnostic and clinical challenges. Haematologica. 2014;99(9):1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansoor A, Pittaluga S, Beck PL, Wilson WH, Ferry JA, Jaffe ES. NK-cell enteropathy: A benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: Clinicopathologic features and follow-up in a unique case series. Blood. 2011;117:1447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia D, Morgan EA, Berger D, Pinkus GS, Ferry JA, Zukerberg LR. NK-cell enteropathy and similar indolent lymphoproliferative disorders: A case series with literature review. Am J Clin Pathol. 2019;151(1):75–85. [DOI] [PubMed] [Google Scholar]
- 7.Terai T, Sugimoto M, Uozaki H, et al. Lymphomatoid gastropathy mimicking extranodal NK/T cell lymphoma, nasal type: A case report. World J Gastroenterol. 2012;18(17):2140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuyoshi T, Mai NH, Tomoko MT, et al. Clinicopathologic analysis of 6 lymphomatoid gastropathy cases. Expanding the disease spectrum to CD4− CD8+ cases. Am Surgpathol. 2015;39(9):1259–66. [DOI] [PubMed] [Google Scholar]