Abstract
Purpose
The objective of this scientometric analysis was to recognize the top 100 cited articles on ‘Male infertility and Antioxidants’ and analyze its publication characteristics.
Materials and Methods
The Scopus database was used to retrieve related articles and the top 100 identified based on citation rate.
Results
The articles were published in 56 journals between 1995 and 2019 with a median (interquartile range) citation score of 17 (5–62). Among the top 100 articles, 69 were clinical studies, which included controlled and blinded (33.33%), prospective (27.54%), randomized-controlled trials (26.09%), uncontrolled (11.59%), and retrospective (1.45%) studies. In addition to conventional semen parameters, advanced sperm function tests such as oxidative stress (51%) and sperm DNA damage (23%) were reported. Pregnancy rate (33%) was found to be the most reported reproductive outcome. Antioxidant therapy was mostly investigated in male cohorts with sperm abnormalities such as asthenozoospermia (28%) and clinical conditions such as idiopathic male infertility (20%), varicocele/varicocelectomy (17%) and general male infertility (16%).
Conclusions
The most influential publications on antioxidants and male infertility were identified for the first time in the literature. This will serve as a reliable source of information for researchers and clinicians alike.
Keywords: Antioxidants; Clinical trial; Infertility, male; Publications; Research
INTRODUCTION
Infertility is defined as the inability of couples to conceive after 12 months of regular sexual intercourse without contraception [1]. Couple infertility is reported to affect as much as 15% of the global population [2], in which male factor is solely responsible for 20% to 70% [3]. Notably, evidence suggests that male factor infertility is significantly increasing over recent decades, with a general decline in semen quality [4,5]. Although conditions such as varicocele, genital tract infection, reproductive tract obstruction and hypogonadism are relatively common causes of male infertility [2], a large proportion of cases remain of unknown origin. Approximately 15% of cases are classified as unexplained male infertility (UMI), defined as patients being infertile despite having normal semen parameters. In addition, another 30% to 50% of the cases are classified as idiopathic male infertility (IMI), defined as the patient being infertile and having one or more abnormal semen parameters without identifiable cause [6,7]. The harmful role of oxidative stress (OS) on male fertility has been widely demonstrated [8,9,10]. Importantly, 30% to 80% of IMI cases have increased OS and are described as ‘Male Oxidative Stress Infertility’ (MOSI) [6].
Studies on the effect of antioxidants on male infertility and sperm functions have been reported since the mid-1990s [11,12]. Significant decrease in seminal antioxidant has been reported in infertile men [13]. The earliest clinical trial investigated vitamin E in a cohort of infertile males, reporting an improvement in semen parameters following 3 months of treatment in a double-blinded cross-over design [11]. Subsequently, numerous studies have investigated the impact of antioxidants on semen characteristics in vitro and in vivo, including sperm parameters, sperm function tests, reactive oxygen species (ROS) concentration, OS markers, and DNA damage alongside reproductive outcomes in natural and assisted reproduction techniques (ART). Currently, there is no clear consensus on the treatment regimen of antioxidants for male infertility [14,15,16,17,18]. A recent Cochrane meta-analysis [19] concluded that antioxidants result in increased pregnancy and live birth rates, with inconclusive outcomes for miscarriage. This report included studies on randomized controlled trials (RCTs), investigating mono or combined antioxidant supplementation in sub-fertile men attending reproductive clinics. However, the studies included were reported as low to very low quality in terms of study design, participant number, and reported outcomes [19].
OS is well-established as a significant contributor to male infertility in both known and unknown causes and underlying risks factors. Antioxidants can serve as a cost-effective, safe and non-invasive therapy in male infertility [18]. Hence, the use of antioxidant therapy in the management of male infertility has been of great interest [6,20]. Numerous articles have been published emphasizing the importance of antioxidant therapy in male infertility. Using the scientometric approach, this study aims to identify the top 100 cited articles in the field of ‘Male Infertility and Antioxidants’ and conduct a detailed analysis of these top cited publications to study its characteristics. This involves analysis of the top cited articles based on year of publication, country of origin, type of publication, main subject area, types of study design and antioxidant formulation employed, clinical conditions investigated, and reproductive outcomes assessed. This will provide important insight into the highly referenced articles in the field of antioxidants and male infertility.
MATERIALS AND METHODS
1. Ethics statement
This study was exempted from the approval of the Institution Review Board (IRB) as it did not involve any human subject and was conducted using the scientometric data retrieved from the Scopus database.
2. Identification of top 100 highly cited articles
The quantitative analysis of scientific literature was performed using the scientometric approach. Scientometrics is the quantitative study of scientific disciplines based on published literature and scientific communication. It may include identification of emerging areas of scientific research, examination of research development over time, and/or its geographical and organizational distribution [21]. The Scopus database is a reliable tool for conducting a scientometrics study [22,23]. To date, the Scopus database has 1.4 billion cited references dating back to 1970 with a rapidly increasing database of over 70,000 indexed articles (https://www.elsevier.com/solutions/scopus/how-scopus-works/content).
The articles of interest were retrieved from Scopus using the function ‘TITLE-ABSTRACT-KEYWORDS’. Specific keyword string was used to retrieve a maximum number of relevant scientific articles on human. The keyword string was composed of TITLE-ABS-KEY (“antioxidant*”) AND TITLE-ABS-KEY (“male infertil*” OR “male subfertil*” OR “male sterility”) AND TITLE-ABS-KEY (“human”). The search and the extraction of articles were performed on March 26, 2020. All articles available in the literature from the start and published until 2019 were retrieved using specific keyword string without setting any restrictions on the language. However, book chapters and conference papers were excluded from the search (Supplement Fig. 1). Then, we calculated the citation rate for all retrieved articles by applying the following formula:
Though citation number is considered as a useful indicator of scientific impact of an article, it may be influenced by the publication year resulting in older manuscripts having a higher number of citations. To nullify this influence, we calculated the citation rate by dividing the number of citations by the number of years since publication [24,25]. Subsequently, we listed the articles in descending order according to their citation rate and selected the top 100 cited articles for our analysis.
The top 100 cited articles were analyzed for publication characteristics such as publication year, journal, subject area, country of origin, institution, and publication type (original research, review, systematic review, and meta-analysis) (Supplement Fig. 1). Furthermore, the study characteristics of original articles such as study type (prospective, cross-sectional, retrospective, uncontrolled, RTCs, controlled and blinded), antioxidant formulation employed (individual, combined, and registered), clinical conditions investigated (idiopathic, oligozoospermic, asthenozoospermic, oligoasthenozoospermic, oligoasthenoteratozoospermic (OAT), varicocele, azoospermia, UMI, and others), sperm parameters (conventional sperm parameters and advanced sperm function tests), and reproductive outcomes assessed (fertilization, pregnancy, miscarriage, implantation, and live birth rate) were also analyzed.
3. Statistical analysis
Scientometric data obtained from the Scopus database based on the data retrieval strategies depicted in the flow chart (Supplement Fig. 1) was saved as comma-separated value (CSV) files and subsequently converted to Microsoft Excel files for descriptive statistical analyses [23]. The citation rate was then calculated using the citation rate formula as stated above using Microsoft Excel 2013 (Microsoft Corporation, Seattle, WA, USA).
RESULTS
1. Distribution of top 100 articles
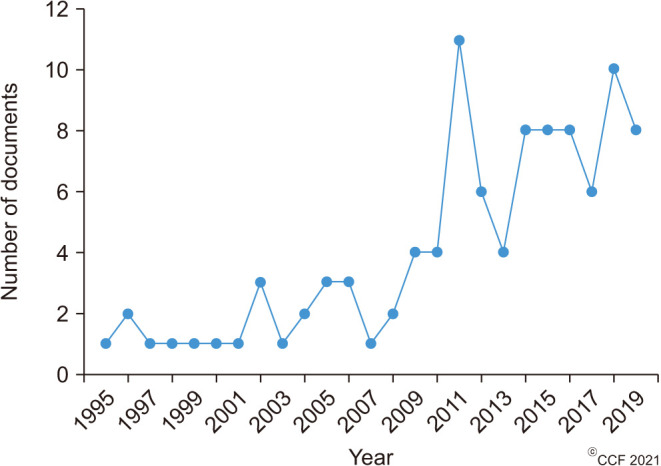
A total of 156 out of 998 articles extracted from the Scopus database were found to be related to male infertility and antioxidants. Table 1 lists the top 100 cited articles based on the citation rate. Furthermore, characteristic analysis of these articles revealed that the review article by Gharagozloo and Aitken (2011), published in Human Reproduction, was the top most cited with a total of 231 citations and a citation rate of 25.7 (Table 1). The top 100 articles analyzed were published during the time period 1995–2019 (Fig. 1). During this time period of 25 years, the median year (interquartile range, IQR) for the top 100 articles was 2007 (2001–2013). The mean (median) number of these top 100 papers before and after 2008 was 1 (1.6) and 7 (6.5), respectively. We also noticed that the number of highly cited articles significantly (p<0.0001) increased after 2008 (Fig. 1), with the greatest number of articles being published in 2011 (n=11) and 2018 (n=10).
Table 1. The top 100 articles investigating male infertility and antioxidants based on the citation rate.
| Rank | Manuscript (first author, title, and year) | Total citations | Citation rate |
|---|---|---|---|
| 1 | Gharagozloo P., The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy, 2011 | 231 | 25.7 |
| 2 | Greco E., Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment, 2005 | 279 | 18.6 |
| 3 | Agarwal A., Role of antioxidants in treatment of male infertility: an overview of the literature, 2004 | 291 | 18.2 |
| 4 | Suleiman S. A., Lipid peroxidation and human sperm motility: protective role of vitamin E, 1996 | 396 | 16.5 |
| 5 | Kodama H., Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients, 1997 | 373 | 16.2 |
| 6 | Showell M. G., Antioxidants for male subfertility, 2014 | 90 | 15 |
| 7 | Ross C., A systematic review of the effect of oral antioxidants on male infertility, 2010 | 143 | 14.3 |
| 8 | Kefer J. C., Role of antioxidants in the treatment of male infertility, 2009 | 142 | 12.9 |
| 9 | Keskes-Ammar L., Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men, 2003 | 204 | 12 |
| 10 | Ahmadi S., Antioxidant supplements and semen parameters: an evidence based review, 2016 | 45 | 11.3 |
| 11 | Safarinejad M. R., Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study, 2011 | 92 | 10.2 |
| 12 | Showell M. G., Antioxidants for male subfertility, 2011 | 89 | 9.9 |
| 13 | Moslemi M. K., Selenium–vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate, 2011 | 82 | 9.1 |
| 14 | Jannatifar R., Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men, 2019 | 9 | 9 |
| 15 | Rolf C., Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with highdose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study, 1999 | 187 | 8.9 |
| 16 | Kessopoulou E., A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility, 1995 | 213 | 8.5 |
| 17 | Busetto G. M., Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-terato-zoospermia, with and without varicocele: a double-blind placebo-controlled study, 2018 | 17 | 8.5 |
| 18 | Lanzafame F. M., Oxidative stress and medical antioxidant treatment in male infertility, 2009 | 92 | 8.4 |
| 19 | Henkel R., The excessive use of antioxidant therapy: a possible cause of male infertility? 2018 | 8 | 8 |
| 20 | Comhaire F. H., The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men, 2000 | 149 | 7.5 |
| 21 | Ghanem H., Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial, 2010 | 71 | 7.1 |
| 22 | Comhaire F. H., Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: a double blind, randomized trial, 2005 | 103 | 6.9 |
| 23 | Bejarano I., Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa, 2014 | 41 | 6.8 |
| 24 | Balercia G., Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia, 2005 | 101 | 6.7 |
| 25 | Lafuente R., Coenzyme Q10 and male infertility: a meta-analysis, 2013 | 46 | 6.6 |
| 26 | Calogero A., Conservative nonhormonal options for the treatment of male infertility: antibiotics, anti-Inflammatory drugs, and antioxidants, 2017 | 18 | 6 |
| 27 | Cardoso J. P., Optimizing male fertility: oxidative stress and the use of antioxidants, 2019 | 6 | 6 |
| 28 | Lombardo F., The role of antioxidant therapy in the treatment of male infertility: an overview, 2011 | 52 | 5.8 |
| 29 | Geva E., The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program, 1996 | 130 | 5.4 |
| 30 | Balercia G., Coenzyme Q10 supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study, 2004 | 85 | 5.3 |
| 31 | Gupta N. P., Lycopene therapy in idiopathic male infertility – a preliminary report, 2002 | 93 | 5.2 |
| 32 | Ciftci H., Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status, 2009 | 56 | 5.1 |
| 33 | Ahmad M. K., Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males, 2010 | 51 | 5.1 |
| 34 | ElSheikh M. G., Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: a prospective, randomized trial, 2015 | 25 | 5 |
| 35 | Alahmar A. T., The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia, 2018 | 10 | 5 |
| 36 | Vicari E., Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds, 2002 | 89 | 4.9 |
| 37 | Shukla K. K., Mucuna pruriens reduces stress and improves the quality of semen in infertile men, 2010 | 49 | 4.9 |
| 38 | Kumar R., Drug therapy for idiopathic male infertility: rationale versus evidence, 2006 | 67 | 4.8 |
| 39 | Gual-Frau J., Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients, 2015 | 23 | 4.6 |
| 40 | Imamovic Kumalic S., Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia, 2014 | 27 | 4.5 |
| 41 | Garg H., An update on the role of medical treatment including antioxidant therapy in varicocele, 2016 | 18 | 4.5 |
| 42 | Safarinejad M. R., The roles of omega-3 and omega-6 fatty acids in idiopathic male infertility, 2012 | 32 | 4 |
| 43 | Safarinejad M. R., Effect of pentoxifylline on semen parameters, reproductive hormones, and seminal plasma antioxidant capacity in men with idiopathic infertility: a randomized double-blind placebo-controlled study, 2011 | 35 | 3.9 |
| 44 | Oliva A., Pentoxifylline and antioxidants improve sperm quality in male patients with varicocele, 2009 | 42 | 3.8 |
| 45 | Condorelli R. A., Effects of myoinositol on sperm mitochondrial function in-vitro, 2011 | 33 | 3.7 |
| 46 | Safarinejad M. R., A prospective double-blind randomized placebo-controlled study of the effect of saffron (Crocus sativus Linn.) on semen parameters and seminal plasma antioxidant capacity in infertile men with idiopathic oligoasthenoteratozoospermia, 2011 | 32 | 3.6 |
| 47 | Mahdavi R., Effects of black seeds (Nigella sativa) on male infertility: a systematic review, 2015 | 18 | 3.6 |
| 48 | Omu A. E., Treatment of asthenozoospermia with zinc sulphate: andrological, immunological and obstetric outcome, 1998 | 77 | 3.5 |
| 49 | Barekat F., A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy, 2016 | 14 | 3.5 |
| 50 | Cyrus A., The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial, 2015 | 17 | 3.4 |
| 51 | Ahmad M. K., Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men, 2008 | 40 | 3.3 |
| 52 | Shukla K. K., Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations, 2011 | 27 | 3 |
| 53 | Nematollahi-Mahani S. N., Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy, 2014 | 16 | 2.7 |
| 54 | Ko E. Y., The role of over-the-counter supplements for the treatment of male infertility-fact or fiction? 2012 | 21 | 2.6 |
| 55 | Lu X. L., Melatonin therapy adds extra benefit to varicocelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: a placebo-controlled, double-blind trial, 2018 | 5 | 2.5 |
| 56 | Patel S. R., Antioxidant therapy in male infertility, 2008 | 27 | 2.3 |
| 57 | Yamamoto Y., The effects of tomato juice on male infertility, 2017 | 7 | 2.3 |
| 58 | Kobori Y., Improvement of seminal quality and sexual function of men with oligoasthenoteratozoospermia syndrome following supplementation with L-Arginine and Pycnogenol®, 2015 | 11 | 2.2 |
| 59 | Busetto G. M., Prospective open-label study on the efficacy and tolerability of a combination of nutritional supplements in primary infertile patients with idiopathic astenoteratozoospermia, 2012 | 16 | 2 |
| 60 | Singh A., To evaluate the efficacy of combination antioxidant therapy on oxidative stress parameters in seminal plasma in the male infertility, 2016 | 7 | 1.8 |
| 61 | Festa R., Coenzyme Q10 supplementation in infertile men with low-grade varicocele: an open, uncontrolled pilot study, 2014 | 10 | 1.7 |
| 62 | Bozhedomov V. A., Using L- and acetyl-L-carnintines in combination with clomiphene citrate and antioxidant complex for treating idiopathic male infertility: a prospective randomized trial, 2017 | 5 | 1.7 |
| 63 | Thakur A. S., Effect of ubiquinol therapy on sperm parameters and serum testosterone levels in oligoasthenozoospermic infertile men, 2015 | 8 | 1.6 |
| 64 | Bosman E., Effect of metformin therapy and dietary supplements on semen parameters in hyperinsulinaemic males, 2015 | 8 | 1.6 |
| 65 | Bolle P., The controversial efficacy of vitamin E for human male infertility, 2002 | 27 | 1.5 |
| 66 | Gudeloglu A., Medical management of male infertility in the absence of a specific etiology, 2014 | 9 | 1.5 |
| 67 | Montanino Oliva M., Effect of myoinositol and antioxidants on sperm quality in men with metabolic syndrome, 2016 | 6 | 1.5 |
| 68 | Durg S., Withania somnifera (Indian ginseng) in male infertility: an evidence-based systematic review and metaanalysis, 2018 | 3 | 1.5 |
| 69 | Fatehi D., Reactive oxygenated species (ROS) in male fertility; source, interaction mechanism and antioxidant therapy, 2018 | 3 | 1.5 |
| 70 | Filipcikova R., Lycopene improves the distorted ratio between AA/DHA in the seminal plasma of infertile males and increases the likelihood of successful pregnancy, 2015 | 7 | 1.4 |
| 71 | Pourmand G., Does l-carnitine therapy add any extra benefit to standard inguinal varicocelectomy in terms of deoxyribonucleic acid damage or sperm quality factor indices: a randomized study, 2014 | 8 | 1.3 |
| 72 | Negri L., Effect of superoxide dismutase supplementation on sperm DNA fragmentation, 2017 | 4 | 1.3 |
| 73 | Alahmar A. T., Effect of vitamin C, vitamin E, zinc, selenium, and coenzyme Q10 in infertile men with idiopathic oligoasthenozoospermia, 2017 | 4 | 1.3 |
| 74 | Khani B., Effect of sesame on sperm quality of infertile men, 2013 | 9 | 1.3 |
| 75 | Hosseini J., The influence of ginger (Zingiber officinale) on human sperm quality and DNA fragmentation: a double-blind randomized clinical trial, 2016 | 5 | 1.3 |
| 76 | Kumar R., Herbo-mineral supplementation in men with idiopathic oligoasthenoteratospermia: a double blind randomized placebo-controlled trial, 2011 | 10 | 1.1 |
| 77 | Safarnavadeh T., Antioxidants and infertility treatment, the role of Satureja Khuzestanica: a mini-systematic review, 2011 | 10 | 1.1 |
| 78 | Gamidov S. I., Adjuvant antioxidant therapy in varicocele infertility, 2017 | 3 | 1 |
| 79 | Gamidov S. I., The role of drug therapy in the management of varicocele, 2018 | 2 | 1 |
| 80 | Stenqvist A., Impact of antioxidant treatment on DNA fragmentation index: a double-blind placebo-controlled randomized trial, 2018 | 2 | 1 |
| 81 | Mahdiani E., Effect of carob (Ceratonia siliqua L.) oral supplementation on changes of semen parameters, oxidative stress, inflammatory biomarkers and reproductive hormones in infertile men, 2018 | 2 | 1 |
| 82 | Nouri M., The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: a randomized, doubleblind, placebo‐controlled clinical trial, 2019 | 1 | 1 |
| 83 | Li M. C., Men's Intake of vitamin C and β-carotene is positively related to fertilization rate but not to live birth rate in couples undergoing infertility treatment, 2019 | 1 | 1 |
| 84 | Gamidov S. I., Double-blind, randomized placebo-controlled study of efficiency and safety of complex acetyl-L-carnitine, L-carnitine fumarate and alpha-lipoic scid (Spermactin Forte) for Treatment of Male Infertility, 2019 | 1 | 1 |
| 85 | Popova A. Y., Antioxidant therapy improves the results of НВА-test in infertile men during a preparation for assisted reproductive technology (IVF/ICSI), 2019 | 1 | 1 |
| 86 | Cannarella R., Non-hormonal treatment for male infertility: the potential role of Serenoa repens, selenium and lycopene, 2019 | 1 | 1 |
| 87 | Gopinath P. M., Fixed dose combination therapy of antioxidants in treatment of idiopathic oligoasthenozoospermia: Results of a randomized, double-blind, placebo-controlled clinical trial, 2013 | 6 | 0.9 |
| 88 | Bardaweel S. K., Alternative and antioxidant therapies used by a sample of infertile males in Jordan: a cross-sectional survey, 2014 | 5 | 0.8 |
| 89 | Vicari E., Antioxidant therapeutic efficiency after the use of carnitine in infertile patients with bacterial or nonbacterial prostato-vesiculo-epididymitis, 2001 | 14 | 0.7 |
| 90 | Yadav S. B., Antioxidant treatment a new therapeutic approach to reversible male infertility, 2006 | 7 | 0.5 |
| 91 | Galimov Sh. N., Effects of L-carnitine on ejaculate parameters in males from infertile couples, 2012 | 4 | 0.5 |
| 92 | Bozhedomov V. A., The use of nutrient complexes in idiopathic male infertility associated with asteno- and/or teratozoospermia: the search of predictors of treatment efficiency (preliminary results), 2018 | 1 | 0.5 |
| 93 | Javadi M., Effect of propolis oral supplements on sperm parameters and oxidative stress indicator in idiopathic infertile men: a double-blind randomized clinical trial, 2018 | 1 | 0.5 |
| 94 | Gamidov C. I., Current approach to therapy for male infertility in patients with varicocele, 2012 | 3 | 0.4 |
| 95 | Verzeletti F. B., Evaluation of sperm quality in adults after use spirulina platensis and resveratrol, 2012 | 3 | 0.4 |
| 96 | Li W., Biological function of CoQ10 and its effect on the quality of spermatozoa, 2006 | 4 | 0.3 |
| 97 | da Silva T. M., Folic acid does not improve semen parametrs in subfertile men: a double-blinded, randomized, placebo-controlled study, 2013 | 2 | 0.3 |
| 98 | Liu W., Astaxanthin in male reproduction: advances in studies, 2016 | 1 | 0.25 |
| 99 | Chattopadhyay R., Effect of continuous 6 months oral antioxidant combination with universally recommended dosage in idiopathic male infertility, 2016 | 1 | 0.25 |
| 100 | Oborná I., Lycopene therapy in male infertility, 2007 | 3 | 0.23 |
Fig. 1. Distribution of top 100 articles based on year of publication.
Citation analysis reported a median (IQR) of 17 (5–62) for the total citations received by the top 100 articles and a median (IQR) of 3.35 (1.2–6.3) when these citations were analyzed per year. Among the top cited articles, the publication by Suleiman et al (1996) received the highest number of citations (396) followed by the articles published by Kodama et al in 1997 (373) and Agarwal et al in 2004 (291) (Table 1).
2. Publication characteristics of top 100 articles
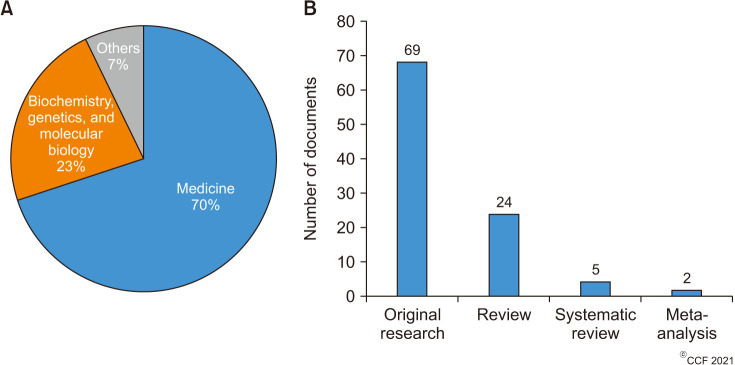
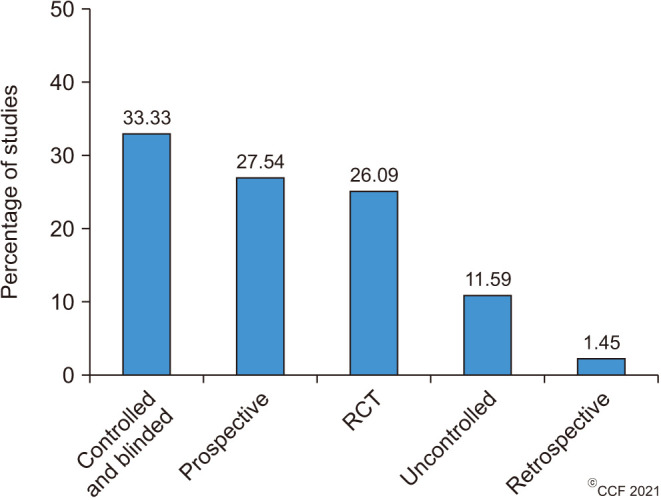
The top 100 cited articles were published by 56 different journals (Table 2), of which Fertility and Sterility (n=10; 2018 Impact factor 5.41) published the highest number of articles, followed by Andrologia (n=7; 2018 Impact factor 1.84), Asian Journal of Andrology (n=4; 2018 Impact factor 2.86), Reproductive Biomedicine Online (n=4; 2018 Impact factor 2.93) and Urologiia Moscow Russia 1999 (n=4; SCImago Journal Rank [SJR] 0.11). Among the top-cited articles, a total of 19 articles originated from Iran (n=19), followed by Italy (n=17) and India (n=14) (Fig. 2). The maximum number of articles was published by two institutions, namely Universitá degli Studi di Catania (n=6) and the National Medical Research Center Obsterics Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russian Federation (n=6), followed jointly by the Cleveland Clinic Foundation (n=5) and Tehran University of Medical Sciences (n=5). The subject areas mainly covered by the top-cited articles were Medicine (70%), Biochemistry, Genetics, and Molecular Biology (23%) while the remaining 7% consisted of others (Fig. 3A). Most of the publications were original research articles (69%), followed by reviews (24%), systematic reviews (5%), and meta-analyses (2%) (Fig. 3B). Furthermore, stratification of original research articles based on the study design revealed that most of the studies were controlled and blinded (n=23, 33.33%), followed by prospective (n=19, 27.54%), RCTs (n=18, 26.09%), uncontrolled (n=8, 11.59%) and retrospective (n=1, 1.45%) studies (Fig. 4).
Table 2. Journals in which the 100 most cited manuscripts were published.
| Journal title | No. of manuscripts published from the top 100 most cited list |
|---|---|
| Fertility and Sterility | 10 |
| Andrologia | 7 |
| Asian Journal of Andrology | 4 |
| Reproductive Biomedicine Online | 4 |
| Urologiia Moscow Russia 1999 | 4 |
| Archivio Italiano Di Urologia E Andrologia | 3 |
| International Journal of Infertility and Fetal Medicine | 3 |
| Journal of Andrology | 3 |
| Andrology | 2 |
| Biomed Research International | 2 |
| European Review for Medical and Pharmacological Sciences | 2 |
| Human Reproduction | 2 |
| International Journal of Reproductive Biomedicine | 2 |
| International Urology and Nephrology | 2 |
| Jornal Brasileiro De Reproducao Assistida | 2 |
| Journal of Clinical and Diagnostic Research | 2 |
| Phytotherapy Research | 2 |
| Urologiia | 2 |
| Urology | 2 |
| Zhonghua Nan Ke Xue National Journal of Andrology | 2 |
| Cochrane Database of Systematic Reviews | 2 |
| Archives of Andrology | 1 |
| Asia Pacific Journal of Clinical Nutrition | 1 |
| BMC Complementary and Alternative Medicine | 1 |
| Biomedical Papers | 1 |
| Biomedical Research | 1 |
| Ceska Gynekologie | 1 |
| Clinical and Experimental Reproductive Medicine | 1 |
| Contraception | 1 |
| European Journal of Obstetrics and Gynecology and Reproductive Biology | 1 |
| Evidence Based Complementary and Alternative Medicine | 1 |
| Human Fertility | 1 |
| Indian Journal of Urology | 1 |
| International Brazilian Journal of Urology | 1 |
| International Journal of Endocrinology | 1 |
| International Journal of Fertility and Sterility | 1 |
| International Journal of General Medicine | 1 |
| International Journal of Urology | 1 |
| Iranian Journal of Obstetrics Gynecology and Infertility | 1 |
| Iranian Journal of Reproductive Medicine | 1 |
| Journal of Assisted Reproduction and Genetics | 1 |
| Journal of Herbal Medicine | 1 |
| Journal of Nutrition | 1 |
| Journal of Pineal Research | 1 |
| Journal of Research in Medical Sciences | 1 |
| Journal of Urology | 1 |
| Phytomedicine | 1 |
| Prostaglandins Leukotrienes and Essential Fatty Acids | 1 |
| Reproductive Biology and Endocrinology | 1 |
| Research Journal of Pharmacy and Technology | 1 |
| Scientific Journal of Kurdistan University of Medical Sciences | 1 |
| Seminars in Reproductive Medicine | 1 |
| Terapevticheskii Arkhiv | 1 |
| Urologic Clinics of North America | 1 |
| Urologii Combining Double Inverted Breve A Moscow Russia 1999 | 1 |
| World Journal of Urology | 1 |
Fig. 2. Top 10 countries that contributed to the 100 most cited publications on male infertility and antioxidants.
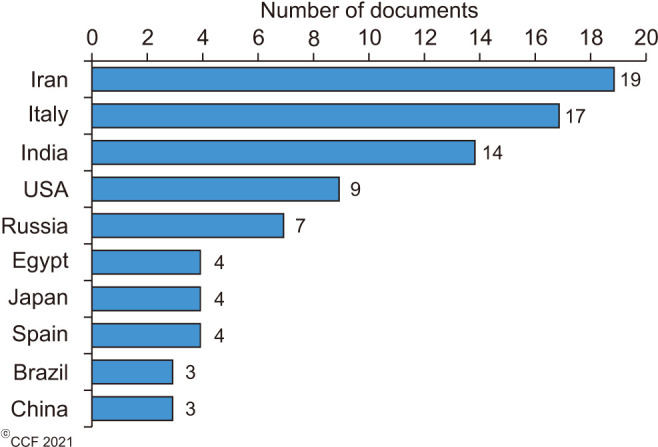
Fig. 3. Manuscript type. (A) Pie chart demonstrating the subject areas mainly covered by the top 100 articles. (B) Bar graph demonstrating the percentage of the 100 most cited articles according to manuscript type.
Fig. 4. Bar diagram demonstrating the distribution of original research articles based on study design (controlled and blinded, n=23; prospective, n=19; randomized controlled trial [RCT], n=18; uncontrolled, n=8; retrospective, n=1).
3. Study characteristics of top cited research articles
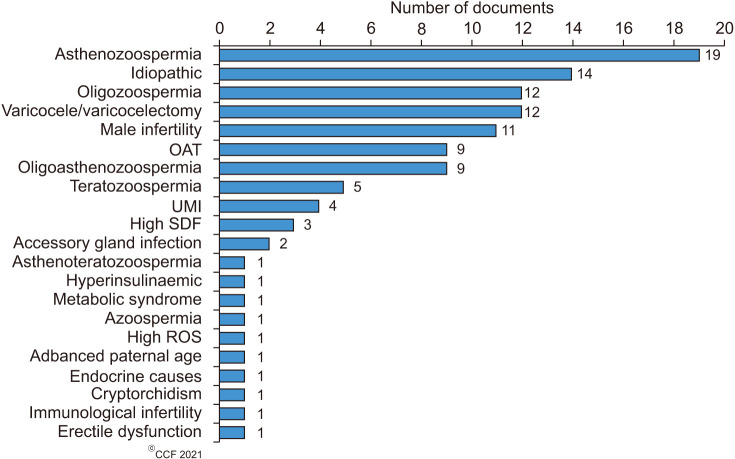
In the current study, we have noticed that 69 of the 100 highly cited articles were original observational clinical studies or clinical trials which have used either individual or combined or registered antioxidants in the treatment of male infertility. Our results revealed that about 46% of the clinical trials used individual antioxidants, followed by 35% combined and 19% registered antioxidant formulations (Fig. 5). Individual antioxidants that were investigated include Vitamin E (n=5), N-acetyl-cysteine, carnitine and lycopene (n=3 each), and co-enzyme Q10, melatonin, vitamin C, and the herbal extract of Withania somnifera and Mucana pruriens (n=2 each) (Supplement Table 1). In addition to Withania somnifera and Mucana pruriens, other herbal extracts that were used as individual antioxidants (n=9) include Ceratonia siliqua (carob), Crocus sativus (saffron), Sesamum indicum (seasame), Zingiber of ficinalis (ginger) and Spirilina platensis (n=1 each). In terms of clinical conditions, asthenozoospermia (n=19, 28%), IMI (n=14, 20%), varicocele/varicocelectomy (n=12, 17%), and general male infertility (n=11, 16%) were the most investigated (Fig. 6).
Fig. 5. Distribution of research articles based on antioxidant formulation investigated.
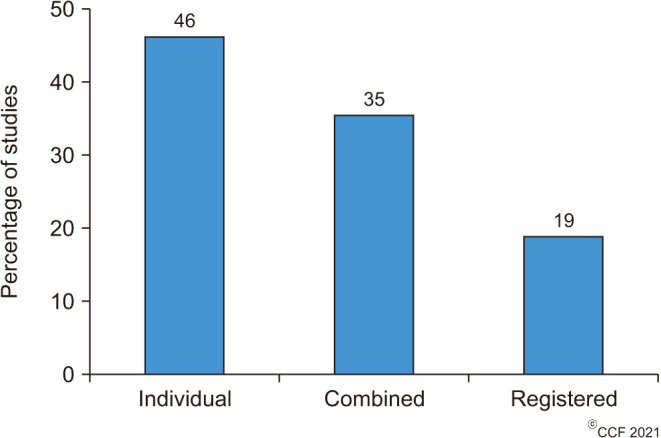
Fig. 6. Conditions associated with male infertility in which antioxidant treatment was most studied. OAT: oligoasthenoteratozoospermic, UMI: unexplained male infertility, SDF: sperm DNA fragmentation, ROS: reactive oxygen species.
The effect of antioxidant treatment on conventional semen parameters, advanced sperm function tests, and reproductive outcomes were also evaluated in the top 100 articles (Table 3). Of the total 69 antioxidant trials, conventional semen parameters (n=64, 93%) and sperm function tests (n=65, 94%) were evaluated in the majority of the studies, whereas reproductive outcomes were assessed in 48% of the studies (n=33). To assess the sperm function, tests to evaluate OS were carried out in 51% of the clinical trials (n=35) and sperm DNA damage testing was conducted in 23% of the studies (n=16). Other sperm functional tests such as capacitation/acrosome reaction (n=3, 4%), zona binding (n=2, 3%), mitochondrial membrane integrity (n=2, 3%) and annexin V binding (n=2, 3%) were employed in very few clinical trials. Additional assays such as sperm-hyaluronic acid binding (HBA) test, hypo-osmotic swelling (HOS) test, and mixed agglutination reaction (MAR) test were performed in only 1% (n=1) of the total clinical studies.
Table 3. Standard semen parameters, advanced sperm function tests, and reproductive outcomes evaluated in trials investigating antioxidant treatment in male infertility (n=69).
| Parameters evaluated | Number of studies | Percentage of studies evaluated | |
|---|---|---|---|
| Standard Semen parameters | 64 | 93 | |
| Sperm function tests | 65 | 94 | |
| Oxidative stress | 35 | 51 | |
| Sperm DNA damage | 16 | 23 | |
| Capacitation/acrosome reaction | 3 | 4 | |
| Zona binding | 2 | 3 | |
| Mitochondrial membrane integrity | 2 | 3 | |
| Annexin V binding | 2 | 3 | |
| Propidium iodide double staining | 2 | 3 | |
| HBA test | 1 | 1 | |
| HOS test | 1 | 1 | |
| MAR test | 1 | 1 | |
| Reproductive outcomes | 33 | 48 | |
| Pregnancy rate | 23 | 33 | |
| Fertilization rate | 4 | 6 | |
| Live birth rate | 3 | 4 | |
| Implantation rate | 1 | 1 | |
| Miscarriage rate | 1 | 1 | |
| Embryo quality | 1 | 1 | |
HBA: sperm-hyaluronic acid binding, HOS: hypo-osmotic swelling, MAR: mixed agglutination reaction.
The most important reproductive outcomes studied include pregnancy, fertilization, implantation live birth and miscarriage rates as well as embryo quality. In 69 of the highly cited research articles, pregnancy rate was the most reported reproductive outcome (n=23, 33%) followed by fertilization rate (n=4, 6%) and live birth rate with 4% (n=3). Implantation rate, miscarriage rate, and embryo quality were reported in only 1% (n=1) of the 69 publications.
DISCUSSION
OS is a well-established cause of sperm dysfunction and it contributes to male infertility [10,26,27,28]. OS develops when the seminal oxidants outweigh the reductants (antioxidants), causing a redox imbalance and thereby negatively affecting male fertility. Antioxidants can counteract OS-induced effects on fertility by augmenting the scavenging capacity of seminal fluid. Hence, a potential approach in the treatment of seminal OS would be to either lower the ROS levels or increase the antioxidant status in these patients [14]. Studies on antioxidant administration have shown some beneficial effects in the management of male infertility, albeit with inconsistent results [17,19,29]. The relative safety and cost-effectiveness of antioxidant supplementation justifies the increased focus of ongoing research towards investigating its contribution in combating male infertility [14]. However, studies are yet to report of conclusive evidence regarding the antioxidants that are most beneficial in the management of male infertility and the clinical conditions in which it would be most favorable.
With increased number of publications in this field, identifying the most referenced articles would be of great significance for researchers and clinicians. Therefore, the objective of this scientometric analysis was to recognize the top 100 cited articles on ‘Male Infertility and Antioxidants’ and analyze its characteristics based on the year of publication, country of origin, institution, type of publication, and study design. The antioxidant formulation mainly investigated, and their association with clinical conditions, semen parameters, and reproductive outcomes were also evaluated.
Of the original articles identified (n=69) in the top 100, 49 were reported as clinical trials (blinded and controlled, n=23; RCT, n=18; uncontrolled, n=8) while 22 were observational studies (prospective, n=19; retrospective, n=3). The importance of these study designs in determining clinical efficacy and effectiveness would explain the dominance of RCTs and observational studies in the top 100 cited articles compared to the various other study designs.
Both observational studies and RCTs are generally the primary study approaches used to investigate new treatments in a clinical context. While each of these designs has its own strengths and limitations, RCTs are traditionally considered more valid due to the additional measure to reduce bias via randomization [30,31,32]. In clinical studies, randomization allocates participants into different comparative groups to reduce baseline differences through appropriate random sequence generation [30,33,34]. The inclusion of blinding involves the concealment of the allocation of a specific group from participants and researchers involved in the study, further increasing its validity [30,31,32]. In fact, the reporting of allocation concealment and randomization methods in RCT is increasingly being standardized and considered mandatory in the literature [33,35].
While RCTs are considered a hallmark of evidence-based medicine [36] and a gold standard for studying causal relationships [37], both RCTs and observational studies may complement each other in evaluating treatment effects [38,39]. Observational studies that are well-designed and well-executed may potentially draw similar conclusions as RCTs, while requiring less resources. This may explain the high proportion of prospective observational studies seen here, which are followed closely by non-blinded RCT studies that have less validity.
The majority of the top-cited research articles investigated individual antioxidant applications (50.72%). Interpretation of causation and efficacy is arguably more appropriate with an individual therapeutic intervention than with combined antioxidants [30,31,32], which may explain the predominance of individual antioxidant trials in the literature. Vitamin E was the most researched individual antioxidant which has been demonstrated to neutralize ROS and reduce lipid peroxidation [40,41,42]. Similarly, there was an even spread of other antioxidants individually investigated, which can be explained as they all have well-demonstrated roles in the protection of sperm from OS [43]. These include carnitines, N-acetyl cysteine, lycopene, co-enzyme Q10, melatonin, vitamin C, zinc, and folic acid [1,42,44,45]. However, the combination of antioxidants may offer synergistic protection against OS [46,47,48], leading to a relatively high proportion of combined products investigated. The combined antioxidant formulation may arguably be more pragmatic and help ameliorate more than one manifestation of infertility such as sperm quality and erectile function [34,49]. A variety of medicinal herbs were found to be investigated as well in these clinical trials. A rise in herbal pharmacognosy is due to the recent resurgence of scientific interest in plant compounds for medicinal use [40], and that is reflected in the studies. Through secondary plant metabolites, including phenols, flavonoids, and tannins [41], plant extracts are well known to have antioxidant activity [44,50]. These antioxidants obtained from herbal extractions may offer benefits in spermatogenesis and sperm protection, improving fertility outcomes in males [45,51]. Therefore, a growing research interest in naturally-available antioxidants from plant sources for male infertility management is expected.
Semen quality is usually evaluated by means of standard semen analysis, which still represents the mainstay in the workup of the male fertility status. It indicates the quality of ejaculated semen and indirectly reflects the functionality of the duct system and accessory glands [1]. Additionally, advanced sperm function tests are currently performed to further investigate the functional status of the sperm [52]. OS leads to oxidation of lipids and proteins as well as impairment of mitochondrial membrane potential. This alters the energy metabolism, causing axonemal dysfunction and significantly affecting sperm morphology and motility [53,54]. OS has also been associated with increased levels of DNA damage [55], with severe consequences on male fertility [56,57,58]. Therefore, OS and sperm DNA damage testing have received much attention in recent years [22] and our results show that both tests were most often used in antioxidant trials.
Improvement in semen quality reflects the efficacy of the antioxidant treatment, hence it is not surprising that evaluation of semen parameters after antioxidant therapy is the main outcome reported in 93% of the top-cited original articles. However, a real positive effect of antioxidant therapy on semen quality is still under debate as studies report contradictory results including improvement [59,60,61] or no changes [62,63,64]. This discrepancy may be due to incorrect patient selection or a different response to the therapy due to different pathophysiological background of the patients included in the study. Yet, on the other hand, the individual interactions and interrelations in terms of the biological action of various antioxidants are not sufficiently known. Therefore, inappropriate concentration ratios of different antioxidants may lead to suboptimal results for a specific group of patients. Furthermore, it is not known whether a specific andrological condition would need a specific antioxidant formulation, specifically addressing the potential deficiency.
Based on our analysis, asthenozoospermic patients are the main target for the antioxidant treatment. This is in agreement with broad evidence reporting the impairment of sperm motility as one of the main OS-related sperm damage manifestations [65,66,67]. ROS-related lipid peroxidation of the sperm membrane significantly alters the flagellar architecture and affects the maintenance of a physiological mitochondrial membrane potential, which is responsible for the energy metabolism and thus sperm motility [68]. Therefore, in this regard, the improvement of sperm motility may be considered as a successful outcome of antioxidant treatment.
In 2019, the term MOSI was proposed to classify those patients showing abnormal semen parameters and high OS, thereby including many infertile patients previously classified as “idiopathic” [6]. Since OS plays a dominant role in the etiology of male infertility, MOSI patients may benefit from the treatment with antioxidants, as recently reported by Arafa et al [69] who selected idiopathic infertile patients with high levels of seminal OS to be treated with antioxidants. Therefore, apart from selecting a suitable combination of antioxidants at the right concentration, a proper patient selection may be a key factor in eliciting the beneficial effects of antioxidant supplementation.
Greco et al (2005), the second top cited article in the field of antioxidants and male infertility, highlighted the reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment [29] in agreement with other reports included in the top cited articles [59,60,61,70]. Therefore, the testing of sperm DNA damage may represent one of the most important advanced sperm function tests, to better reflect sperm quality.
The efficacy of any antioxidant treatment is evaluated based on the reproductive outcomes. The World Health Organization (WHO) guidelines define “fertility” as “the capacity to establish a pregnancy” [71] hence, the pregnancy rate is the main outcome to be assessed for fertility evaluation. Preliminary data supports the use of specific antioxidant combinations in clinical practice as there has been an increased rate of spontaneous or ART-related pregnancy reported [62,63]. Some of the top cited articles in the present study have also reported an improvement in the pregnancy rate [63,64,72], suggesting that antioxidant treatment is beneficial in treating male infertility. It is also important to highlight that despite live birth rate being the most important reproductive outcome in human reproduction, it is addressed in only 4% of the clinical trials. This could be one of the main reasons for uncertainty on the use of antioxidants in the treatment of male infertility.
CONCLUSIONS
For the first time, our study reports the scientometric data of the top 100 articles investigating antioxidants and male infertility. Analysis of top-cited articles revealed asthenozoospermia and idiopathic infertility as the primary groups targeted for antioxidant clinical trials and pregnancy rate as the most reported reproductive outcome. Furthermore, this study disseminates useful scientific knowledge to both researchers and clinicians regarding the use of antioxidants in treatment of OS-mediated male infertility.
The present study illustrates the significant heterogeneity underpinning the debatable use of antioxidants as a therapeutic modality in improving male infertility. This is evidenced by the wide range of different antioxidant regimens investigated, either individually or combined, in the top 100 articles on male infertility and antioxidants in the past 25 years. There was also little consistency in the relevant reproductive outcomes and underlying clinical conditions that were investigated.
Even though the range of redox balance in human semen at normal physiological level was recently reported [73], the cut-off value is still unknown. As such, the optimal preparation of antioxidants needed to maintain the delicate redox balance in various conditions that impair fertility is still a matter of investigation. Furthermore, it is imperative to understand more clearly about how antioxidants penetrate the bloodtestis and blood-epididymis barriers, and interact with the Sertoli and Leydig cells, and the developing germ cells, as well as at which antioxidant concentration and composition. These essential information need be elucidated in order to develop effective treatments that address the physiological requirements of specific patient groups such as those with asthenozoospermia, UMI, or IMI.
ACKNOWLEDGEMENTS
Graphic artists from Cleveland Clinic's Arts and Medical Photography Department helped in the manuscript illustrations.
Financial support for this study was provided by the American Center for Reproductive Medicine, Cleveland Clinic, Ohio, USA.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: AA, SB, RH.
- Data analysis: MKPS, RF, CB, KAR, RFA, CI.
- Supervision: SB, KL, DD, PNP, RH.
- Writing — original draft: All the authors.
- Writing — review & editing: All the authors.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.200181.
The distribution and frequency of individual antioxidants (n=22) used in original articles (n=69) investigating antioxidants in male infertility
Flow diagram displaying the analysis of top cited articles in the field of male infertility and antioxidants.
References
- 1.WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- 2.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–882. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23:646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male Oxidative Stress Infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37:296–312. doi: 10.5534/wjmh.190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada A, Esteves SC, Nizza M, Agarwal A. Unexplained male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576–594. doi: 10.1590/s1677-55382012000500002. [DOI] [PubMed] [Google Scholar]
- 8.Dada R, Bisht S. In: Male infertility: understanding, causes and treatment. Singh R, Singh K, editors. Singapore: Springer Singapore; 2017. Oxidative stress and male infertility; pp. 151–165. [Google Scholar]
- 9.Alahmar AT. Role of oxidative stress in male infertility: an updated review. J Hum Reprod Sci. 2019;12:4–18. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol. 2017;16:35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–831. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 12.Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–537. [PubMed] [Google Scholar]
- 13.Lazzarino G, Listorti I, Muzii L, Amorini AM, Longo S, Di Stasio E, et al. Low-molecular weight compounds in human seminal plasma as potential biomarkers of male infertility. Hum Reprod. 2018;33:1817–1828. doi: 10.1093/humrep/dey279. [DOI] [PubMed] [Google Scholar]
- 14.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–124. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Hidalgo D, Bragado MJ, Batista AR, Oliveira PF, Alves MG. Antioxidants and male fertility: from molecular studies to clinical evidence. Antioxidants (Basel) 2019;8:89. doi: 10.3390/antiox8040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed. 2016;14:729–736. [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso JP, Cocuzza M, Elterman D. Optimizing male fertility: oxidative stress and the use of antioxidants. World J Urol. 2019;37:1029–1034. doi: 10.1007/s00345-019-02656-3. [DOI] [PubMed] [Google Scholar]
- 19.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leisegang K, Henkel R. In: Male infertility in reproductive medicine: diagnosis and management. Rizk B, Agarwal A, Sabanegh ES Jr, editors. Boca Raton (FL): CRC Press; 2019. Oxidative stress: relevance, evaluation, and management; pp. 119–128. [Google Scholar]
- 21.Maity A, Teli S. Growth of literature in Higgs Boson: a scientometric analysis of SCOPUS database (2005-2014) Libr Philos Pract [Internet] 2015:1272. Available from: http://digitalcommons.unl.edu/libphilprac/1272. [Google Scholar]
- 22.Baskaran S, Agarwal A, Panner Selvam MK, Finelli R, Robert KA, Iovine C, et al. Tracking research trends and hotspots in sperm DNA fragmentation testing for the evaluation of male infertility: a scientometric analysis. Reprod Biol Endocrinol. 2019;17:110. doi: 10.1186/s12958-019-0550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal A, Baskaran S, Panner Selvam MK, Barbăroșie C, Master K. Unraveling the footsteps of proteomics in male reproductive research: a scientometric approach. Antioxid Redox Signal. 2020;32:536–549. doi: 10.1089/ars.2019.7945. [DOI] [PubMed] [Google Scholar]
- 24.Ellul T, Bullock N, Abdelrahman T, Powell AG, Witherspoon J, Lewis WG. The 100 most cited manuscripts in emergency abdominal surgery: a bibliometric analysis. Int J Surg. 2017;37:29–35. doi: 10.1016/j.ijsu.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Bullock N, Ellul T, Bennett A, Steggall M, Brown G. The 100 most influential manuscripts in andrology: a bibliometric analysis. Basic Clin Androl. 2018;28:15. doi: 10.1186/s12610-018-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Durairajanayagam D, Halabi J, Peng J, Vazquez-Levin M. Proteomics, oxidative stress and male infertility. Reprod Biomed Online. 2014;29:32–58. doi: 10.1016/j.rbmo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Aitken RJ. Oxidative stress and the etiology of male infertility. J Assist Reprod Genet. 2016;33:1691–1692. doi: 10.1007/s10815-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 29.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–353. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 30.Stanley K. Design of randomized controlled trials. Circulation. 2007;115:1164–1169. doi: 10.1161/CIRCULATIONAHA.105.594945. [DOI] [PubMed] [Google Scholar]
- 31.Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008;1:211–217. doi: 10.1016/j.jcin.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Probst P, Grummich K, Heger P, Zaschke S, Knebel P, Ulrich A, et al. Blinding in randomized controlled trials in general and abdominal surgery: protocol for a systematic review and empirical study. Syst Rev. 2016;5:48. doi: 10.1186/s13643-016-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. New York (NY): John Wiley & Sons; 2011. [Google Scholar]
- 34.Gamerman V, Cai T, Elsäßer A. Pragmatic randomized clinical trials: best practices and statistical guidance. Health Serv Outcomes Res Methodol. 2019;19:23–35. [Google Scholar]
- 35.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32 [Google Scholar]
- 36.Spieth PM, Kubasch AS, Penzlin AI, Illigens BM, Barlinn K, Siepmann T. Randomized controlled trials - a matter of design. Neuropsychiatr Dis Treat. 2016;12:1341–1349. doi: 10.2147/NDT.S101938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125:1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014;(4):MR000034. doi: 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol. 2016;16:102. doi: 10.1186/s12871-016-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy Upton RH. In: Evidence-based validation of herbal medicine. Mukherjee PK, editor. Amsterdam: Elsevier; 2015. Traditional herbal medicine, pharmacognosy, and pharmacopoeial standards: a discussion at the crossroads; pp. 45–85. [Google Scholar]
- 41.Gutzeit HO, Ludwig-Müller J. In: Plant natural products: synthesis, biological functions and practical applications. Gutzeit HO, Ludwig-Müller J, editors. Weinheim: Wiley-VCH; 2014. Biosynthesis and chemical properties of natural substances in plants; pp. 1–79. [Google Scholar]
- 42.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–723. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Lazzarino G, Listorti I, Bilotta G, Capozzolo T, Amorini AM, Longo S, et al. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants (Basel) 2019;8:96. doi: 10.3390/antiox8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabera JN, Semana E, Mussa AR, He X. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol. 2014;2:377–392. [Google Scholar]
- 45.Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuno F, Tanimukai S, Sasaki M, Ikejima C, Yamashita F, Kodama C, et al. Combination of antioxidant supplements improved cognitive function in the elderly. J Alzheimers Dis. 2012;32:895–903. doi: 10.3233/JAD-2012-121225. [DOI] [PubMed] [Google Scholar]
- 47.Truscott TG. Synergistic effects of antioxidant vitamins. Bibl Nutr Dieta. 2001;(55):68–79. doi: 10.1159/000059464. [DOI] [PubMed] [Google Scholar]
- 48.Crespo YA, Bravo Sánchez LR, Quintana YG, Cabrera AST, Bermúdez Del Sol A, Mayancha DMG. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon. 2019;5:e01942. doi: 10.1016/j.heliyon.2019.e01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobori Y, Suzuki K, Iwahata T, Shin T, Sadaoka Y, Sato R, et al. Improvement of seminal quality and sexual function of men with oligoasthenoteratozoospermia syndrome following supplementation with L-arginine and Pycnogenol®. Arch Ital Urol Androl. 2015;87:190–193. doi: 10.4081/aiua.2015.3.190. [DOI] [PubMed] [Google Scholar]
- 50.Piasecka A, Jedrzejczak-Rey N, Bednarek P. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 2015;206:948–964. doi: 10.1111/nph.13325. [DOI] [PubMed] [Google Scholar]
- 51.Mohammadi F, Nikzad H, Taherian A, Amini Mahabadi J, Salehi M. Effects of herbal medicine on male infertility. Anat Sci J. 2013;10:3–16. [Google Scholar]
- 52.Talwar P, Hayatnagarkar S. Sperm function test. J Hum Reprod Sci. 2015;8:61–69. doi: 10.4103/0974-1208.158588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–1640. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003;80(Suppl 2):844–850. doi: 10.1016/s0015-0282(03)00983-x. [DOI] [PubMed] [Google Scholar]
- 55.Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17:87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–2917. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 57.Jin J, Pan C, Fei Q, Ni W, Yang X, Zhang L, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril. 2015;103:910–916. doi: 10.1016/j.fertnstert.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2010;16:30–36. doi: 10.1093/molehr/gap080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gual-Frau J, Abad C, Amengual MJ, Hannaoui N, Checa MA, Ribas-Maynou J, et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum Fertil (Camb) 2015;18:225–229. doi: 10.3109/14647273.2015.1050462. [DOI] [PubMed] [Google Scholar]
- 60.Cyrus A, Kabir A, Goodarzi D, Moghimi M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: a double blind placebo controlled clinical trial. Int Braz J Urol. 2015;41:230–238. doi: 10.1590/S1677-5538.IBJU.2015.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu XL, Liu JJ, Li JT, Yang QA, Zhang JM. Melatonin therapy adds extra benefit to varicecelectomy in terms of sperm parameters, hormonal profile and total antioxidant capacity: a placebo-controlled, double-blind trial. Andrologia. 2018;50:e13033. doi: 10.1111/and.13033. [DOI] [PubMed] [Google Scholar]
- 62.Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant "Astaxanthin" treatment for male infertility: a double blind, randomized trial. Asian J Androl. 2005;7:257–262. doi: 10.1111/j.1745-7262.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 63.Filipcikova R, Oborna I, Brezinova J, Novotny J, Wojewodka G, De Sanctis JB, et al. Lycopene improves the distorted ratio between AA/DHA in the seminal plasma of infertile males and increases the likelihood of successful pregnancy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:77–82. doi: 10.5507/bp.2013.007. [DOI] [PubMed] [Google Scholar]
- 64.Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-astheno-teratozoospermia, with and without varicocele: a double-blind placebo-controlled study. Andrologia. 2018;50:e12927. doi: 10.1111/and.12927. [DOI] [PubMed] [Google Scholar]
- 65.Ferramosca A, Pinto Provenzano S, Montagna DD, Coppola L, Zara V. Oxidative stress negatively affects human sperm mitochondrial respiration. Urology. 2013;82:78–83. doi: 10.1016/j.urology.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 66.Dobrakowski M, Kasperczyk S, Horak S, Chyra-Jach D, Birkner E, Kasperczyk A. Oxidative stress and motility impairment in the semen of fertile males. Andrologia. 2017;49:e12783. doi: 10.1111/and.12783. [DOI] [PubMed] [Google Scholar]
- 67.Mahfouz R, Sharma R, Thiyagarajan A, Kale V, Gupta S, Sabanegh E, et al. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil Steril. 2010;94:2141–2146. doi: 10.1016/j.fertnstert.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 68.Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction. 2020;159:R189–R201. doi: 10.1530/REP-19-0452. [DOI] [PubMed] [Google Scholar]
- 69.Arafa M, Agarwal A, Majzoub A, Panner Selvam MK, Baskaran S, Henkel R, et al. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants (Basel) 2020;9:219. doi: 10.3390/antiox9030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol. 2019;17:24. doi: 10.1186/s12958-019-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32:1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–2235. doi: 10.1016/j.fertnstert.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 73.Panner Selvam MK, Agarwal A, Henkel R, Finelli R, Robert KA, Iovine C, et al. The effect of oxidative and reductive stress on semen parameters and functions of physiologically normal human spermatozoa. Free Radic Biol Med. 2020;152:375–385. doi: 10.1016/j.freeradbiomed.2020.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The distribution and frequency of individual antioxidants (n=22) used in original articles (n=69) investigating antioxidants in male infertility
Flow diagram displaying the analysis of top cited articles in the field of male infertility and antioxidants.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).