Abstract
Introduction
This study aimed to assess the association between fasting serum C-peptide levels and the presence of impaired awareness of hypoglycemia (IAH) in people with type 1 diabetes.
Research design and methods
We performed a cross-sectional study among 509 individuals with type 1 diabetes (diabetes duration 5–65 years). Extensive clinical data and fasting serum C-peptide concentrations were collected and related to the presence or absence of IAH, which was evaluated using the validated Dutch version of the Clarke questionnaire. A multivariable logistic regression model was constructed to investigate the association of C-peptide and other clinical variables with IAH.
Results
In 129 (25%) individuals, residual C-peptide secretion was detected, while 75 (15%) individuals reported IAH. The median (IQR) C-peptide concentration among all participants was 0.0 (0.0–3.9) pmol/L. The prevalence of severe hypoglycemia was lower in people with demonstrable C-peptide versus those with absent C-peptide (30% vs 41%, p=0.025). Individuals with IAH were older, had longer diabetes duration, more frequently had macrovascular and microvascular complications, and more often used antihypertensive drugs, antiplatelet agents and cholesterol-lowering medication. There was a strong association between IAH and having a severe hypoglycemia in the preceding year. In multivariable regression analysis, residual C-peptide, either continuously or dichotomous, was associated with lower prevalence of IAH (p=0.040–0.042), while age at diabetes onset (p=0.001), presence of microvascular complications (p=0.003) and body mass index (BMI) (p=0.003) were also independently associated with the presence of IAH.
Conclusions
Higher BMI, the presence of microvascular complications and higher age at diabetes onset were independent risk factors for IAH in people with type 1 diabetes, while residual C-peptide secretion was associated with lower risk of this complication.
Keywords: awareness, C-peptide, hypoglycemia, age of onset
Significance of this study.
What is already known about this subject?
Impaired awareness of hypoglycemia (IAH) may develop with longer duration of diabetes.
Previous studies have reported an association between preserved C-peptide secretion and lower risk of (severe) hypoglycemia.
What are the new findings?
Higher body mass index, the presence of microvascular complications and higher age at diabetes onset were independent risk factors for IAH in people with type 1 diabetes.
There was a strong association between IAH and having a severe hypoglycemia in the preceding year.
Residual C-peptide secretion was protective, both for IAH and for severe hypoglycemia (multivariable model).
How might these results change the focus of research or clinical practice?
Even low residual C-peptide secretion has beneficial clinical effect in people with type 1 diabetes.
Introduction
Impaired awareness of hypoglycemia (IAH) is a serious consequence of long-standing diabetes mellitus. It is defined as the inability to detect the onset of hypoglycemia1 2 and thereby the perception of hypoglycemia is attenuated or even absent. IAH develops in approximately 20%–40% of individuals with type 1 diabetes mellitus and 15%–20% of insulin-treated individuals with type 2 diabetes, and lower intensity of symptoms and higher prevalence of IAH increase with longer duration of diabetes.3–5 Earlier studies showed that IAH is associated with an increased risk of asymptomatic and severe hypoglycemia6–9 and has a strong negative impact on quality of life.2 10 Furthermore, cognitive impairment, seizure, coma and even death may occur.11–13
Residual C-peptide secretion can be found in approximately 30%–80% of people with long-term type 1 diabetes.14 Previous studies have shown an association between preserved C-peptide secretion and the glucagon response to hypoglycemia,15 as well as a reduced frequency of self-reported hypoglycemic episodes.16–20 Individuals with type 1 diabetes who lacked C-peptide secretion had a fourfold increased risk of severe hypoglycemia.21 As the presence of C-peptide was associated with a lower rate of hypoglycemia, it can be hypothesized that residual C-peptide secretion may also be associated with the absence of IAH. An earlier study by Holstein et al, in a cohort of 217 individuals with type 1 diabetes, showed that longer diabetes duration, C-peptide status and lower glycated hemoglobin (HbA1c) were risk factors for the presence of impaired hypoglycemia awareness.22 The aim of the present study was to evaluate to which extent C-peptide and other clinical variables are related to the presence of IAH in a large cohort of individuals with type 1 diabetes.
Research design and methods
Study design
The current study is a cross-sectional analysis of data derived from the ‘Biomarkers of heterogeneity in type 1 diabetes’ project, a collaboration between Diabeter, Haaglanden Medical Center (HMC) and the University Medical Center Groningen (UMCG). For the present study, we used baseline data collected between January 2016 and May 2019. The study was approved by the Medical Ethical Review Committee of the UMCG (METC 2015/493). All participants provided written informed consent.
Study population
Individuals with type 1 diabetes were included between June 2016 and March 2019. Inclusion criteria were a clinical diagnosis of type 1 diabetes, a diabetes duration ≥5 years and age ≥16 years. Presence or absence of IAH was not a criterion for participation. In total, this prospective cohort study included individuals with type 1 diabetes. The baseline evaluation comprised the collection of diabetes-related complaints, comorbidity and complications, medical treatment, laboratory measurements and a dedicated questionnaire. Additional data were collected from the electronic charts and included demographics (age, gender, age at onset, duration of diabetes), physical measurements (height, weight, systolic and diastolic blood pressure, body mass index (BMI)), laboratory data, clinical data (mode of insulin administration, including continuous subcutaneous insulin infusion (CSII)), glucose measurements (including intermittently scanned or continuous glucose monitoring), medication use, alcohol intake, and data on the presence of microvascular and macrovascular complications.
Microvascular complications included microalbuminuria, macroalbuminuria, retinopathy and neuropathy. Microalbuminuria was defined as 20–200 mg/L albumin or an albumin/creatinine ratio between 3.5 and 35 mg/mmol in women and between 2.5 and 25 mg/mmol in men. Macroalbuminuria was defined as >200 mg/L albumin or an albumin/creatinine ratio >35 mg/mmol in women and >25 mg/mmol in men. Retinopathy was scored by an ophthalmologist and was defined according to national guidelines. Self-reported neuropathy scores were obtained from the Diabetes Neuropathy DN4 questionnaire.23 The presence of microvascular complications was evaluated using a window of 1 year before and after the baseline visit. Presence of macrovascular complications was defined as (a history of) heart failure, transient ischemic attack or cerebrovascular accident, presence of angina pectoris or myocardial infarction, a previous coronary interventions (either coronary artery bypass grafting or percutaneous transluminal coronary angioplasty).
For the current study, the presence of IAH was assessed using the validated Dutch version of the Clarke questionnaire. This questionnaire comprises five questions on symptoms and frequency of hypoglycemia: each positive answer scored one point. A total score of three or more indicates IAH.24 25 Severe hypoglycemia was defined by a positive answer to one of both questions regarding severe hypoglycemia.25
Laboratory measurements
Blood samples for serum C-peptide measurements were collected in the fasting state, between 8:00 and 10:00 in the morning in 482 of the participants, while 27 only had a light breakfast snack. C-peptide concentrations in the latter group were similar to those who were fasting. Routine laboratory data including HbA1c, lipid profile, serum creatinine and urinary albumin excretion were either collected on the same day, or obtained from medical records within the last year. Estimated glomerular filtration rate (eGFR) was calculated as described earlier.26 C-peptide was measured by an immunoradiometric assay (IM3639, Beckman Coulter, Brea, California, USA). The limit of quantitation was 3.8 pmol/L, and interassay coefficient of variation (c.v.) was 9.1% at 6.5 pmol/L.
Statistical analysis
Descriptive continuous data were expressed as mean with SD (±SD) or as median and IQR for normally distributed and non-normally distributed data, respectively. Categorical data were presented as number and percentage (%). Baseline characteristics were compared with the χ2 test for categorical data, and t-test or Mann-Whitney U test for continuous variables, depending on their distribution.
Logistic regression analysis was used to test the association between the presence of IAH and individual parameters, with stepwise forward selection with entry testing based on the significance of the score statistic, and removal testing based on the probability of the Wald statistic. Log transformation was done for variables not-normally distributed. Participants with fasting C-peptide <300 pmol/L were included in the models, as individuals with higher C-peptide concentrations may not have typical type 1 diabetes. Alcohol, also an expected risk factor for IAH,27 was not included in the model due to incomplete data. eGFR was taken into account in the multivariable models because C-peptide is subject to renal excretion.28 Three models were evaluated based on (1) Log-transformed C-peptide; (2) presence or absence of C-peptide (< or ≥3.8 pmol/L); (3) categorical C-peptide, defined as either not detectable, 3.8–20 pmol/L and >20 pmol/L concentration. As a sensitivity analysis, we repeated these analyses with exclusion of the 27 participants who were not completely fasted at blood draw. We also analyzed the contribution of variables to the presence of IAH based on the model of Holstein et al, excluding the KCNJ11 polymorphisms.22 For all analyses, a significance level of p<0.05 was used. Statistical analyses were performed using SPSS (IBM. Released 2015. IBM SPSS Statistics for Windows, V.23.0. IBM).
Results
Complete data for evaluation were available in 509 individuals with type 1 diabetes. Baseline characteristics of the participants stratified by level of IAH are presented in tables 1 and 2.
Table 1.
Characteristics of the participants
| Impaired awareness of hypoglycemia | P value | ||
| Absent | Present | ||
| n=434 | n=75 | ||
| Age, years | 28 (22–51) | 49 (31–59) | <0.001 |
| Female gender, n (%) | 256 (59.0) | 46 (61.3) | 0.702 |
| Diabetes duration, years | 17 (12–29) | 25 (15–39) | <0.001 |
| Diabetes duration >35 years, n (%) | 68 (15.7) | 22 (29.3) | 0.004 |
| Age at diabetes onset, years | 12 (8–19) | 17 (11–27) | 0.001 |
| Age at diabetes onset >18, years, n (%) | 120 (27.6) | 35 (46.7) | 0.001 |
| Body mass index, kg/m2 | 25.0 (22.8–27.6) | 25.6 (24.1–29.3) | 0.007 |
| Insulin pump use, n (%) | 279 (64.3) | 45 (60.0) | 0.476 |
| Insulin dose (U/day) | 55±22 | 50±21 | 0.085 |
| Glucose sensor use, n (%) | 88 (20.3) | 32 (42.7) | <0.001 |
| rtCGM/isCGM (n) | 68/20 | 30/2 | <0.001 |
| Alcohol use, n (%)* | 123 (35.3) | 15 (29.4) | 0.405 |
| HbA1c, % | 7.7 (1.1) | 7.7 (1.0) | 0.994 |
| HbA1c, mmol/mol | 61 (12) | 61 (11) | 0.995 |
| C-peptide continuous, pmol/L | 0.0 (0.0–4.5) | 0.0 (0.0–0.0) | 0.135 |
| C-peptide categorical, <3.8, 3.8–20, >20 pmol/L, n (%) | 319/55/60 (73.5/12.7/13.8) | 61/7/7 (81.4/9.3/9.3) | 0.352 |
| eGFR, mL/min/1.73 m2 | 105±21 | 95±23 | <0.001 |
| Total cholesterol, mmol/L | 4.5 (4.0–5.0) | 4.5 (4.1–5.2) | 0.463 |
| HDL cholesterol, mmol/L | 1.5 (1.3–2.0) | 1.7 (1.4–2.0) | 0.104 |
| LDL cholesterol, mmol/L | 2.5 (2.2–3.0) | 2.5 (2.2–3.1) | 0.820 |
| Triglycerides, mmol/L | 0.8 (0.6–1.2) | 0.9 (0.7–1.2) | 0.201 |
| Albumin:creatinine ratio, mg/mmol | 0.4 (0.0–1.1) | 0.4 (0.0–1.2) | 0.843 |
Data are presented as mean±SD, median (IQR) or n (%).
*Only available in 399 participants.
eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; isCGM, intermittently scanned (flash) glucose monitoring; LDL, low-density lipoprotein; rtCGM, real-time continuous glucose monitoring.
Table 2.
Complications and medication use in the participants
| Impaired awareness of hypoglycemia | P value | ||
| Absent | Present | ||
| n=434 | n=75 | ||
| Self-reported severe hypoglycemia in the past 12 months, n (%) | 136 (31.3) | 58 (77.3) | <0.001 |
| Self-reported severe hypoglycemia requiring medical intervention, n (%) | 19 (4.4) | 22 (29.3) | <0.001 |
| Hypertension, n (%) | 59 (13.6) | 20 (26.7) | 0.004 |
| Any microvascular complication, n (%) | 198 (45.6) | 51 (68.0) | <0.001 |
| Microalbuminuria, n (%) | 66 (15.6) | 15 (20.0) | 0.346 |
| Macroalbuminuria, n (%) | 9 (2.1) | 0 (0.0) | 0.202 |
| Retinopathy, n (%) | 113 (26.0) | 27 (36.5) | 0.063 |
| Neuropathy self-reported, n (%) | 106 (24.4) | 27 (36.0) | 0.035 |
| Macrovascular complications, n (%) | 20 (4.6) | 9 (12.0) | 0.011 |
| Angina pectoris, n (%) | 6 (1.4) | 0 (0.0) | 0.306 |
| MI, n (%) | 7 (1.6) | 5 (6.7) | 0.008 |
| PTCA, n (%) | 6 (1.4) | 5 (6.4) | 0.004 |
| CABG, n (%) | 5 (1.2) | 4 (5.3) | 0.011 |
| TIA/CVA, n (%) | 7 (1.6) | 3 (4.0) | 0.169 |
| Antihypertensive drugs, n (%) | 90 (20.7) | 27 (36.0) | 0.004 |
| Beta-blockers, n (%) | 20 (4.6) | 7 (9.3) | 0.092 |
| Diuretics, n (%) | 27 (6.2) | 14 (18.7) | <0.001 |
| Calcium antagonists, n (%) | 20 (4.6) | 5 (6.7) | 0.446 |
| RAAS blockers, n (%) | 71 (16.4) | 20 (26.7) | 0.031 |
| Cholesterol-lowering drugs, n (%) | 95 (21.9) | 28 (37.3) | 0.004 |
| Antiplatelet agents, n (%) | 23 (5.3) | 9 (12.0) | 0.027 |
| Antidepressants, n (%) | 21 (4.8) | 8 (10.7) | 0.044 |
Data are presented as mean±SD, median (IQR) or n (%).
CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; RAAS, renin-angiotensin-aldosterone system; TIA, transient ischemic attack.
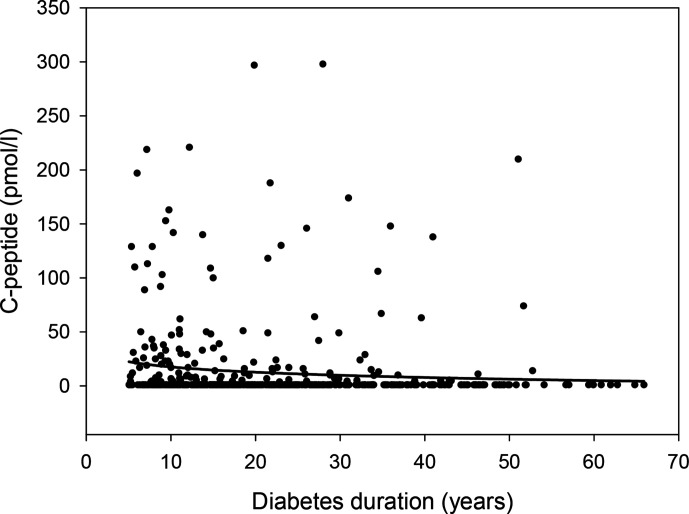
The median age of the study cohort (IQR) was 32 (23–53) years, 302 (59%) individuals were female, the diabetes duration was 19 (12–30) years and age at diabetes onset was 12 (8–20) years. Furthermore, 249 (49%) individuals had at least one microvascular complication and 29 (6%) individuals had a macrovascular complication. In total, 324 (64%) participants administered insulin by continuous subcutaneous insulin infusion using a pump. Mean HbA1c concentration (SD) was 60 (12) mmol/mol/7.7 (1.1)%. In 129 (25%) individuals, residual C-peptide secretion was detected; median C-peptide concentration among all participants was 0.0 (0.0–3.9) pmol/L, 62 (12.2%) had C-peptide concentrations between 3.8 and 20 pmol/L, and 67 (13.2%) had C-peptide concentrations >20 pmol/L. C-peptide concentration decreased with longer duration of diabetes (figure 1).
Figure 1.
Relationship between C-peptide and diabetes duration in 509 individuals with type 1 diabetes. Regression line shows logarithmic fit.
Severe hypoglycemia in the past 12 months was reported by 196 participants (38.5%), 41 (8.1%) of whom required medical intervention. The prevalence of severe hypoglycemia was similar across sex and not related to poorer glycemic control, longer diabetes duration, the presence of microvascular or macrovascular complications, or use of beta-blockers or antidepressants. However, prevalence of severe hypoglycemia was lower in people with demonstrable C-peptide versus those with absent C-peptide (30% vs 41%, p=0.025).
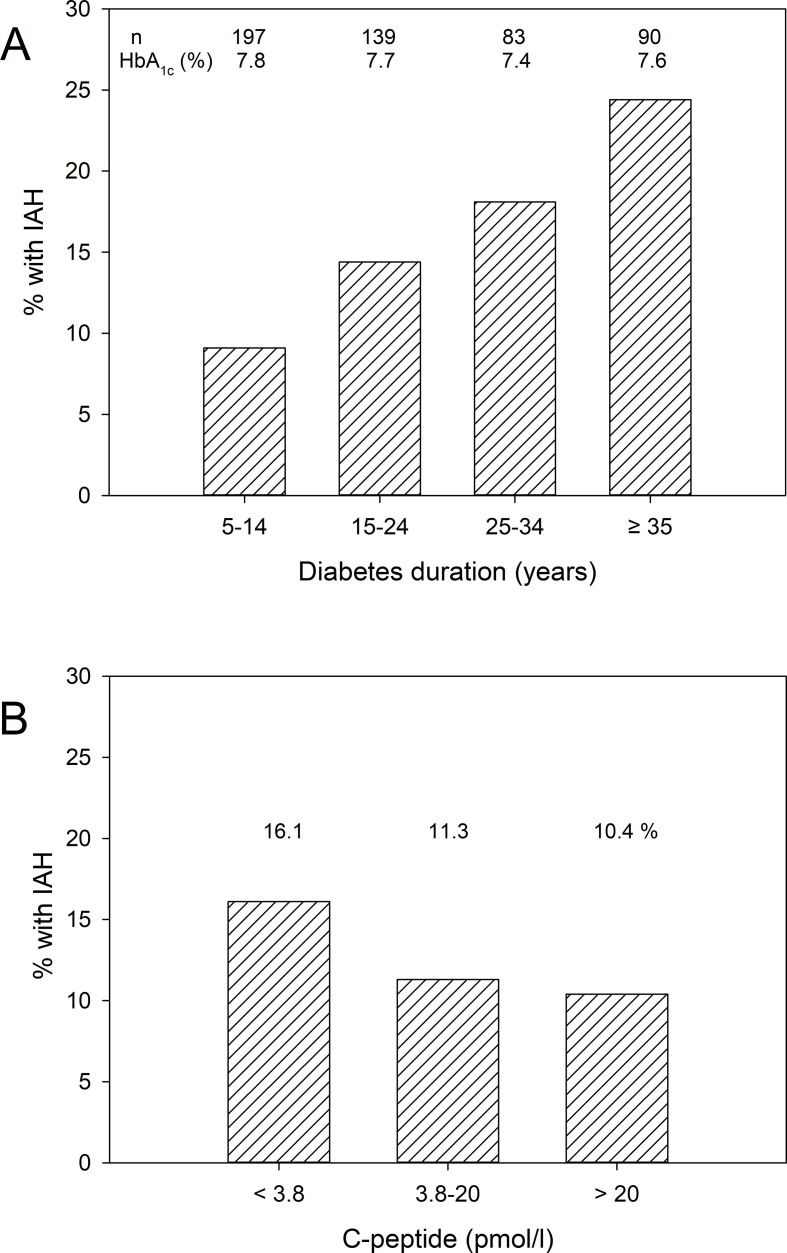
The median value of the modified Clarke score was 1.0 (0.0–2.0). In total, 75 (14.7%) individuals had a modified Clarke score of ≥3 and were classified as having IAH. As demonstrated in table 1, participants with IAH were older, had longer diabetes duration, a higher age of diabetes onset, and significantly more often hypertension, microvascular complications and macrovascular complications. Figure 2A shows the relationship between longer diabetes duration and higher prevalence of IAH, which amounted to 25% in those with diabetes duration >35 years, while figure 2B shows the uncorrected prevalence of IAH in relation to serum C-peptide levels. People with IAH were significantly more commonly treated with antihypertensive drugs, cholesterol-lowering drugs and antiplatelet agents compared with people without IAH (table 2). There was no significant difference in HbA1c and C-peptide concentrations between people with and without IAH. Among people with IAH, 58 (77.3%) reported at least one episode of severe hypoglycemia in the preceding year, and 22 (29.3%) had a severe hypoglycemic incident necessitating medical intervention (table 2). Thus, IAH was associated with a nine times higher risk (OR 9, 1, 95% CI 4.6 to 17.8, p<0.001) of having a severe hypoglycemia necessitating medical intervention in the preceding year.
Figure 2.
(A) Prevalence of impaired awareness of hypoglycemia (IAH) in relation to diabetes duration and HbA1c concentrations. Overall p for % with IAH by χ2=0.006. (B) Prevalence of IAH in relation to serum C-peptide levels. Overall p for % with IAH by χ2=0.352.
In the multivariable logistic regression models, higher C-peptide was significantly associated with the absence of IAH, while especially age at onset, BMI and presence of microvascular complications showed a positive association with the presence of IAH (table 3 and online supplemental tables 1 and 2). The sensitivity analyses, in those who were fasting at blood draw, yielded similar results: model 1, log C-peptide OR 0.63 (0.40 to 0.98), p=0.040; model 2, C-peptide dichotomous OR 0.51 (0.26 to 0.98), p=0.042). We reproduced the analysis published by Holstein et al in 2005 (online supplemental table 3)22 and show that residual C-peptide secretion was negatively associated with IAH, when assessed in this model that also included age, HbA1c, and short and long duration of diabetes.
Table 3.
Multivariable association of impaired awareness of hypoglycemia with clinical parameters, C-peptide continuous log transformed
| Full model | Forward stepwise model | |||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Diabetes duration | 1.02 | (0.99 to 1.04) | 0.261 | |||
| Log age at onset of diabetes, years | 4.01 | (1.63 to 9.86) | 0.003 | 3.99 | (1.77 to 9.01) | 0.001 |
| BMI, kg/m2 | 1.07 | (1.02 to 1.13) | 0.011 | 1.08 | (1.03 to 1.14) | 0.003 |
| Hypertension | 1.20 | (0.63 to 2.32) | 0.576 | |||
| Microvascular complications | 1.83 | (1.00 to 3.33) | 0.048 | 2.28 | (1.34 to 3.90) | 0.003 |
| Macrovascular complications | 1.52 | (0.56 to 4.18) | 0.414 | |||
| Log C-peptide, pmol/L | 0.66 | (0.42 to 1.02) | 0.062 | 0.63 | (0.40 to 0.98) | 0.040 |
| eGFR (CKD-EPI), mL/min/1.73 m2 | 1.00 | (0.99 to 1.02) | 0.954 | |||
| Beta-blocker use | 0.74 | (0.26 to 2.17) | 0.589 | |||
BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate.
bmjdrc-2021-002288supp001.pdf (14.9KB, pdf)
bmjdrc-2021-002288supp002.pdf (85.7KB, pdf)
bmjdrc-2021-002288supp003.pdf (83.4KB, pdf)
Discussion
In this cross-sectional study among 509 individuals with type 1 diabetes, the overall prevalence of IAH was 15%. Longer duration of diabetes, higher age, older age at diabetes onset, higher body mass index and lower eGFR were associated with presence of IAH, as well as the presence of microvascular and macrovascular complications, and a severe hypoglycemia in the preceding year. In multivariable analyses, the presence of residual C-peptide secretion was negatively associated with a prevalence of IAH, while the presence of microvascular complications, higher BMI and older age at onset were positively associated with the presence of IAH. These data confirm the results of previous studies and indicate that even in these modern times, IAH can be a major consequence of long-standing diabetes.
Two previous studies addressed the relationship of C-peptide and IAH. In a retrospective cohort study among 167 persons with type 1 diabetes, no difference could be demonstrated in the presence of IAH, measured using the Gold questionnaire, between groups with (defined as 10–200 pmol/L) and without (<10 pmol/L) C-peptide secretion.19 However, flash glucose monitoring data (Freestyle Libre, Abbott, Witney, UK) showed that time below range and number of low-glucose events were lower in individuals with preserved C-peptide, and low-C-peptide individuals were more likely to report not always being aware of hypoglycemia.19 Although the Clarke and the Gold questionnaires have good concordance,29 differences in instruments use (ie, questionnaires and glucose measurement techniques) should be considered when comparing the results of studies on IAH. It should also be noted that Gibb et al used random and not fasting C-peptide measurements in their statistical analyses.19 Some people with type 1 diabetes who are considered secretors of C-peptide may have a definite increase of C-peptide after a mixed meal.30 Holstein et al demonstrated in a cohort of 217 individuals that the absence of C-peptide was independently associated with IAH. However, their statistical model was confined to the presence or absence of C-peptide, age, HbA1c, long versus short duration of diabetes, and KCNJ11 polymorphisms.22 We were able to replicate their findings by assessing the same parameters in the model, although we could not evaluate the association with the described KCNJ11 polymorphisms.
The prevalence of IAH (15%) in our study employing the validated Dutch version of the Clarke questionnaire25 is lower compared with previous studies. In adults with type 1 diabetes, a prevalence of 19% was reported using the Gold questionnaire,7 while in an online survey, 23% of 418 participants reported impaired awareness, while 15% had uncertain awareness.31 As the incidence of IAH increases with age and diabetes duration,3 4 27 the lower prevalence of IAH in our study may be explained by our relatively young study population (median age of 32 years) as compared with the mean age of 48 years in the study of Geddes et al.7 The relation between older age and longer diabetes duration and IAH is also confirmed by our findings, and 25% of those with >35 years duration of diabetes reported IAH.
It has to be emphasized that actual hypoglycemia rates may influence the association of C-peptide with the presence of IAH. Frequent hypoglycemia or earlier severe hypoglycemia is an important risk factor for IAH.5 Indeed, there was a strong association between presence of IAH and having a severe hypoglycemia necessitating medical intervention in the preceding year. Furthermore, persistent C-peptide secretion and thus preserved endogenous insulin production is only associated with reduced hypoglycemia and not HbA1c.18 This could indicate that C-peptide influences only hypoglycemia and not long-term glycemic outcome measured by HbA1c, suggesting added value to include hypoglycemia rates into the analysis. However, it is possible that there is another pathophysiologic mechanism underlying the association between C-peptide and hypoglycemia versus C-peptide and IAH. Furthermore, presence of C-peptide and the levels of glucose also depend on the intensity of insulin treatment.16 17 It could therefore be argued that the amount of daily insulin units should also have been included in the regression models. However, this is a parameter that is prone to considerable variation depending on, for instance, nutritional factors and differences in physical activity.
As opposed to expected, age at onset was positively (and not negatively) associated with the presence of IAH. In people with type 1 diabetes onset at an early age, autoimmune response against beta-cells is fiercer and persistent C-peptide secretion is less often present. As such, younger age at diabetes onset results in an increased risk of diabetes-related complications.18 32 33 It may be suggested that the presence of IAH is also associated with younger age at diabetes onset. However, in previous research, the probability of developing retinopathy was lower when type 1 diabetes was diagnosed before the age of 5 compared with the age groups 5–11 and >11 years. This could also apply to IAH.34 Duration of diabetes was no longer associated with the presence of IAH in the multivariable model, as longer diabetes duration was also associated with higher prevalence of microvascular complication, lower C-peptide levels and higher BMI, other factors associated with IAH presence (table 3).
In our study, beta-blocker use was not associated with IAH, as demonstrated by others.27 This may be explained by our relatively young study population and limited use of this type of medication. Beta-blocker use has been associated with increased rate of severe hypoglycemia in adults with diabetes.35 Especially in people with IAH, beta-blockers may less often be prescribed because clinicians may fear the effect they can have on attenuating the symptoms of hypoglycemia.2 However, a recent study suggested that beta-blocker use was not related to hypoglycemia unawareness, or burden in hospitalized high-risk insulin-requiring people with diabetes.36 The use of a glucose sensor was higher in participants with IAH. It should be taken into consideration that in The Netherlands individuals with type 1 diabetes and IAH are eligible for both the prescription and the reimbursement of real-time glucose monitoring. Therefore, we consider the use of a glucose sensor a consequence of and not a risk factor for IAH, and did not include this parameter in the multivariable analyses. Finally, BMI was positively associated with the presence of IAH. No previous data on the association of BMI and IAH are available. It can be hypothesized that frequent hypoglycemia may lead to increased caloric intake and thereby higher BMI, as may be regular preventive ‘snacking’ to prevent hypoglycemia. Additionally, a strong impediment for physical exercise in people with type 1 diabetes is fear of severe hypoglycemia,37 a common adverse event of physical exercise in individuals with type 1 diabetes,38 which may also be a factor contributing to higher BMI.
In the present study, we did not observe any difference in HbA1c levels between people with and without IAH. Glycemic impact, displayed by HbA1c, may be a risk factor for the presence of IAH. On one hand, individuals with IAH may have lower HbA1c levels compared with those without IAH because of the fact that tighter glycemic control is associated with more frequent hypoglycemic episodes and loss of awareness as a consequence.39 On the other hand, those with IAH—and their caregivers—may aim to achieve slightly higher HbA1c levels in order to avoid hypoglycemia and therefore more often are hyperglycemic, resulting in an increased risk for developing diabetes-related complications.14 40 Unfortunately, the current study lacks long-term HbA1c measurements to evaluate this further.
Study limitations
Strengths of this study include the multicenter design, large sample size and considerable phenotyping of the study population. A limitation of this study is that no conclusion can be drawn concerning causality of our findings due to its cross-sectional design. A number of variables like alcohol consumption had missing data, which could have influenced the accuracy of its association with IAH. Finally, it can be hypothesized that some people with IAH are unaware of the fact that they do not notice hypoglycemic episodes and therefore under-report hypoglycemia using questionnaires, as such continuous glucose measurements using a sensor would have been of added value.
Conclusions
This study demonstrates that residual C-peptide secretion is associated with lower prevalence of IAH in people with type 1 diabetes. Furthermore, we demonstrated that higher age at onset, presence of microvascular complications and higher BMI were independently associated with the presence of IAH.
Acknowledgments
The authors would like to thank all participants and acknowledge the work of laboratory technicians at the participating hospitals and the IJsselland Hospital. CEV is supported by the MD-PhD program of the University of Groningen and the UMCG. PRvD holds a personal development grant of the European Foundation for the Study of Diabetes (EFSD).
Footnotes
Contributors: MJW, CEV, BHRW and PRvD researched data and performed data analysis. LSMB supervised the C-peptide measurements. MMCdVV and PD were responsible for data collection and validation. GN advised on questionnaires. PHGD, BHRW and HJA were responsible for individual clinic care. MJW and PRvD drafted the initial version of the manuscript. All coauthors revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding: This study was supported by the Juvenile Diabetes Research Foundation (JDRF), grant no. 3-SRA-2014-291-M-R, and the Dutch Diabetes Research Foundation, project 2015.16.1856, for which we are very grateful.
Competing interests: PD, MMCdVV, GN and HJA are employed at Diabeter Netherlands, an independent clinic which was acquired by Medtronic. The research presented here was independently performed and there are no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. All data requests will be subject to relevant GDPR and ethics considerations.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 2005;54:3592–601. 10.2337/diabetes.54.12.3592 [DOI] [PubMed] [Google Scholar]
- 2.Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes 2015;6:912–26. 10.4239/wjd.v6.i7.912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pramming S, Thorsteinsson B, Bendtson I, et al. Symptomatic hypoglycaemia in 411 type 1 diabetic patients. Diabet Med 1991;8:217–22. 10.1111/j.1464-5491.1991.tb01575.x [DOI] [PubMed] [Google Scholar]
- 4.Olsen SE, Asvold BO, Frier BM, et al. Hypoglycaemia symptoms and impaired awareness of hypoglycaemia in adults with type 1 diabetes: the association with diabetes duration. Diabet Med 2014;31:1210–7. 10.1111/dme.12496 [DOI] [PubMed] [Google Scholar]
- 5.McNeilly AD, McCrimmon RJ. Impaired hypoglycaemia awareness in type 1 diabetes: lessons from the lab. Diabetologia 2018;61:743–50. 10.1007/s00125-018-4548-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703. 10.2337/diacare.17.7.697 [DOI] [PubMed] [Google Scholar]
- 7.Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med 2008;25:501–4. 10.1111/j.1464-5491.2008.02413.x [DOI] [PubMed] [Google Scholar]
- 8.Schopman JE, Geddes J, Frier BM. Frequency of symptomatic and asymptomatic hypoglycaemia in type 1 diabetes: effect of impaired awareness of hypoglycaemia. Diabet Med 2011;28:352–5. 10.1111/j.1464-5491.2010.03203.x [DOI] [PubMed] [Google Scholar]
- 9.Rankin D, Elliott J, Heller S, et al. Experiences of hypoglycaemia unawareness amongst people with type 1 diabetes: a qualitative investigation. Chronic Illn 2014;10:180–91. 10.1177/1742395313513911 [DOI] [PubMed] [Google Scholar]
- 10.Davis RE, Morrissey M, Peters JR, et al. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin 2005;21:1477–83. 10.1185/030079905X61929 [DOI] [PubMed] [Google Scholar]
- 11.MacLeod KM. Hypoglycaemia unawareness: causes, consequences and treatment. J R Coll Physicians Lond 2000;34:245–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Shaefer C, Hinnen D, Sadler C. Hypoglycemia and diabetes: increased need for awareness. Curr Med Res Opin 2016;32:1479–86. 10.1185/03007995.2016.1163255 [DOI] [PubMed] [Google Scholar]
- 13.Hansen TI, Olsen SE, Haferstrom ECD, et al. Cognitive deficits associated with impaired awareness of hypoglycaemia in type 1 diabetes. Diabetologia 2017;60:971–9. 10.1007/s00125-017-4233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. The Lancet 2018;391:2449–62. 10.1016/S0140-6736(18)31320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenz S, Mader JK, Regittnig W, et al. Impact of C-peptide status on the response of glucagon and endogenous glucose production to induced hypoglycemia in T1DM. J Clin Endocrinol Metab 2018;103:1408–17. 10.1210/jc.2017-01836 [DOI] [PubMed] [Google Scholar]
- 16.Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The diabetes control and complications trial research group. Ann Intern Med 1998;128:517–23. 10.7326/0003-4819-128-7-199804010-00001 [DOI] [PubMed] [Google Scholar]
- 17.Lachin JM, McGee P, Palmer JP, et al. Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes 2014;63:739–48. 10.2337/db13-0881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marren SM, Hammersley S, McDonald TJ, et al. Persistent C-peptide is associated with reduced hypoglycaemia but not HbA1c in adults with longstanding type 1 diabetes: evidence for lack of intensive treatment in UK clinical practice? Diabet Med 2019;36:1092–9. 10.1111/dme.13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibb FW, McKnight JA, Clarke C, et al. Preserved C-peptide secretion is associated with fewer low-glucose events and lower glucose variability on flash glucose monitoring in adults with type 1 diabetes. Diabetologia 2020;63:906–14. 10.1007/s00125-020-05099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyam A, Colhoun H, McGurnaghan S, et al. Clinical impact of residual C-peptide secretion in type 1 diabetes on glycemia and microvascular complications. Diabetes Care 2021;44:390–8. 10.2337/dc20-0567 [DOI] [PubMed] [Google Scholar]
- 21.Mühlhauser I, Overmann H, Bender R, et al. Risk factors of severe hypoglycaemia in adult patients with type I diabetes--a prospective population based study. Diabetologia 1998;41:1274–82. 10.1007/s001250051065 [DOI] [PubMed] [Google Scholar]
- 22.Holstein A, Plaschke A, Stumvoll M, et al. The Glu23Lys polymorphism in KCNJ11 and impaired hypoglycaemia awareness in patients with type 1 diabetes. J Hum Genet 2005;50:530–3. 10.1007/s10038-005-0288-y [DOI] [PubMed] [Google Scholar]
- 23.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 24.Janssen MM, Snoek FJ, Heine RJ. Assessing impaired hypoglycemia awareness in type 1 diabetes: agreement of self-report but not of field study data with the autonomic symptom threshold during experimental hypoglycemia. Diabetes Care 2000;23:529–32. 10.2337/diacare.23.4.529 [DOI] [PubMed] [Google Scholar]
- 25.van Meijel LA, de Vegt F, Abbink EJ, et al. High prevalence of impaired awareness of hypoglycemia and severe hypoglycemia among people with insulin-treated type 2 diabetes: the dutch diabetes pearl cohort. BMJ Open Diabetes Res Care 2020;8:e000935. 10.1136/bmjdrc-2019-000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab 2010;36 Suppl 3:S64–74. 10.1016/S1262-3636(10)70470-5 [DOI] [PubMed] [Google Scholar]
- 28.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med 2013;30:803–17. 10.1111/dme.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geddes J, Wright RJ, Zammitt NN, et al. An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diabetes Care 2007;30:1868–70. 10.2337/dc06-2556 [DOI] [PubMed] [Google Scholar]
- 30.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: JOSLIN Medalist study. Diabetes 2010;59:2846–53. 10.2337/db10-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conget I, Ávila D, Giménez M, et al. Impaired awareness of hypoglycaemia in subjects with type 1 diabetes. Results of an online survey in a diabetes web site. Endocrinol Nutr 2016;63:121–5. 10.1016/j.endonu.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care 2012;35:465–70. 10.2337/dc11-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392:477–86. 10.1016/S0140-6736(18)31506-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care 2004;27:955–62. 10.2337/diacare.27.4.955 [DOI] [PubMed] [Google Scholar]
- 35.Pathak RD, Schroeder EB, Seaquist ER, et al. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. integrated health care delivery systems: 2005-2011. Diabetes Care 2016;39:363–70. 10.2337/dc15-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long C, Dungan K. Hypoglycemia awareness and burden among hospitalized patients at high risk for hypoglycemia. J Diabetes Complications 2020;34:107521. 10.1016/j.jdiacomp.2019.107521 [DOI] [PubMed] [Google Scholar]
- 37.Colberg SR, Laan R, Dassau E, et al. Physical activity and type 1 diabetes: time for a rewire? J Diabetes Sci Technol 2015;9:609–18. 10.1177/1932296814566231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burr JF, Shephard RJ, Riddell MC. Physical activity in type 1 diabetes mellitus: assessing risks for physical activity clearance and prescription. Can Fam Physician 2012;58:533–5. [PMC free article] [PubMed] [Google Scholar]
- 39.Palerm CC, Willis JP, Desemone J, et al. Hypoglycemia prediction and detection using optimal estimation. Diabetes Technol Ther 2005;7:3–14. 10.1089/dia.2005.7.3 [DOI] [PubMed] [Google Scholar]
- 40.Lind M, Pivodic A, Svensson A-M, et al. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 2019;366:l4894. 10.1136/bmj.l4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2021-002288supp001.pdf (14.9KB, pdf)
bmjdrc-2021-002288supp002.pdf (85.7KB, pdf)
bmjdrc-2021-002288supp003.pdf (83.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request. All data requests will be subject to relevant GDPR and ethics considerations.