Abstract
Introduction
Progression to symptomatic Alzheimer disease (AD) occurs slowly over a series of preclinical stages. Declining functional mobility may be an early indicator of loss of brain network integration and may lead to an increased risk of experiencing falls. It is unknown whether measures of functional mobility and falls are preclinical markers of AD. The purpose of this study is to examine (1) the relationship between falls and functional mobility with AD biomarkers to determine when falls occur within the temporal progression to symptomatic Alzheimer disease, and (2) the attentional compared with perceptual/motor systems that underlie falls and functional mobility changes seen with AD.
Methods and analysis
This longitudinal cohort study will be conducted at the Knight Alzheimer Disease Research Center. Approximately 350 cognitively normal participants (with and without preclinical AD) will complete an in-home visit every year for 4 years. During each yearly assessment, functional mobility will be assessed using the Performance Oriented Mobility Assessment, Timed Up and Go, and Timed Up and Go dual task. Data regarding falls (including number and severity) will be collected monthly by self-report and confirmed through interviews. This study will leverage ongoing neuropsychological assessments and neuroimaging (including molecular imaging using positron emission tomography and MRI) performed by the Knight Alzheimer Disease Research Center. Relationships between falls and biomarkers of amyloid, tau and neurodegeneration will be evaluated.
Ethics and dissemination
This study was approved by the Washington University in St. Louis Institutional Review Board (reference number 201807135). Written informed consent will be obtained in the home prior to the collection of any study data. Results will be published in peer-reviewed publications and presented at national and international conferences.
Trial registration number
NCT04949529; Pre-results.
Keywords: dementia, adult neurology, neurological injury, neuropathology, neurophysiology
Strengths and limitations of this study.
This study is the first to examine whether changes in falls and functional mobility, in conjunction with concurrent brain network changes, can predict progression to Alzheimer disease (AD) in older adults.
This longitudinal study design will enable us to measure falls and functional mobility over 4 years with a well-characterised cohort of 350 community-dwelling older adults who at baseline are cognitively normal (with and without preclinical AD).
Participants receive a comprehensive in-home evaluation of their fall risks and functional mobility, the results of which are shared with each participant.
Older adults may not be compliant with fall monitoring over time.
It may be difficult to differentiate falling from age-related phenotypes such as frailty.
Introduction
Alzheimer disease (AD) is a slowly progressive neurodegenerative disease that affects 60%–70% of the over 50 million people living with dementia worldwide.1 2 Progression to symptomatic AD occurs slowly through a series of preclinical stages marked by changes in molecular biomarkers that can be quantified by neuroimaging, cerebrospinal fluid (CSF) or plasma measures.3 Cognitively normal (CN) stage 0 individuals have no biomarker abnormalities. CN stage 1 individuals have only cerebral amyloidosis, CN stage 2 individuals have amyloidosis and neurodegeneration, and CN stage 3 individuals have evidence of amyloidosis, neurodegeneration and subtle cognitive changes.4–7 These preclinical stages of AD develop over decades and are considered clinically silent.3 However, emerging evidence suggests that impaired functional mobility (gait and balance) and subsequent falls8 may precede symptomatic cognitive impairment.3 9 Declining functional mobility and increases in falls may be due to subtle changes in attention, executive, motor and sensory processing, and may be an early indicator of loss of integration between the central (CNS) and peripheral nervous systems (PNS).8 10–12
Falls are a leading cause of injury, long-term disability, premature institutionalisation and injury-related death in older individuals.13 14 Individuals with symptomatic AD have a 60%–80% increased risk of falling, and those who fall are five times more likely to be institutionalised than similar individuals who do not fall.13 15 A knowledge gap exists as to whether functional mobility and falls could serve as preclinical markers of AD.16
We previously demonstrated that falls occur at higher rates during the preclinical phase of AD, and the mechanisms that underlie the deterioration of cognitive function were associated with declines in gait and balance necessary for functional mobility.9 Functional connections in the brain, referred to as resting state functional connectivity (rs-fc), decrease in symptomatic AD.17 We observed a decrease in rs-fc for CN individuals with preclinical AD in the dorsal attention network (DAN), a set of brain regions involved in attentional control and planning.17 Functional connections both within the DAN and across other resting state networks (RSNs) may affect one’s functional mobility when attempting to navigate home and community environments. While self-reported performance is obtained from CN individuals (with and without preclinical AD), performance-based measures of everyday function are not recorded. Additional research is therefore needed to examine the relationship between functional mobility/falls and rs-fc, especially for CN individuals with preclinical AD.
For this longitudinal observational study, we will evaluate CN individuals (with and without preclinical AD) at baseline who are currently undergoing comprehensive clinical, neuropsychological and biomarker evaluations at the Knight Alzheimer Disease Research Center (Knight ADRC). Annually, we will conduct an in-home evaluation of fall risks and functional mobility and prospective ascertainment of falls. Comparisons of objective assessments of functional mobility will be performed with regard to measures of brain pathology (using in vivo markers of cerebral amyloidosis and neurodegeneration) to allow us to characterise when changes in falls and functional mobility occur during the preclinical stages of AD. We will also examine attentional compared with perceptual/motor systems that underlie falls and functional mobility in preclinical AD. Falls and functional mobility measures could serve as innovative, inexpensive screening tools to identify individuals at increased risk for progression to symptomatic AD. This may have important implications for the timing of interventions in secondary prevention trials in AD and for the development of more precise, effective treatments for individuals with AD.18
Methods and analysis
Participants
In this longitudinal cohort study, community-dwelling older adults will be recruited from an existing cohort followed by the Knight ADRC. Inclusion criteria for this study are as follows: ≥65 years of age, CN (Clinical Dementia Rating (CDR)19 score of 0, indicating no dementia), and collection of biomarkers (CSF) and/or neuroimaging (positron emission tomography (PET) and/or MRI) within 2 years of study enrolment. Recruitment procedures for the Knight ADRC have been published previously.20
Recruitment
Participants (N=350) will be recruited for in-home visits near the time of their annual clinical assessment at the Knight ADRC. Knight ADRC staff will approach participants who meet inclusion criteria about their interest regarding this study. If interested, potential participants will be referred to a study team member who will provide a detailed description of the study procedures and invite the individual to participate. Letters will also be sent to all eligible individuals to invite them to participate in this study. Written, informed consent will be obtained in the home prior to the collection of any study data. This study was approved by the Institutional Review Board at Washington University in St. Louis (reference number: 201807135).
Study procedures
All Knight ADRC participants in principle complete longitudinal clinical and neuropsychological assessment and biomarker studies of biofluids (blood, CSF) and neuroimaging (amyloid PET, structural and functional MRI; see grey boxes in figure 1). For this study, participants additionally will receive an annual in-home visit and will report falls prospectively for the duration of the study (see blue boxes in figure 1).
Figure 1.
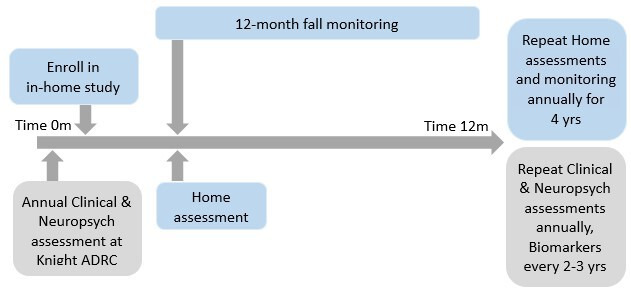
Research design overview. Measures of interest collected by the Knight Alzheimer Disease Research Center (Knight ADRC) will be available at no cost. In-home assessments will be collected annually, and falls will be monitored prospectively.
Knight ADRC Clinical Assessment21
Knight ADRC participants complete an annual clinical assessment battery administered by an experienced clinician using a standardised protocol. During this visit, the CDR assesses the participant’s cognitive and functional performance: 0=CN, 0.5=very mild symptomatic AD, 1=mild symptomatic AD, 2=moderate symptomatic AD or 3=severe symptomatic AD.19 A neurological examination is performed for each participant. At enrolment, participants must have a CDR=0.
Knight ADRC Psychometric/Neuropsychological Assessments22
Participants complete a standard 2-hour psychometric battery within 2 weeks of their annual clinical assessment by an experienced psychometrist and board-certified neurologist blinded to the participant’s preclinical AD status.22 A sensitive composite of attentional and executive control tests that is highly predictive of the transition from healthy ageing to symptomatic AD23–25 will be compared with functional mobility and fall measures.22
Biomarker acquisition/brain neuropathology assessments26
Participants also complete PET scans27 and MRI28 and undergo CSF and blood collection29 30 at the Knight ADRC every 3 years.
PET imaging
PET imaging will be conducted on a 3T Siemens Biograph mMR hybrid scanner using the radiotracer [18F] Florbetapir (AV45) to detect in vivo presence of amyloid in the brain.27 Quantitative image analysis will be performed using a standard amyloid imaging analysis protocol26 that uses FreeSurfer regions of interest (ROIs; Martinos Center for Biomedical Imaging, Charlestown, Massachusetts, USA). Regional standardised uptake value ratios (SUVRs) will be obtained using the cerebellum as the reference region.
Structural MRI
High-resolution structural MRI scans will be acquired using a T1-weighted magnetisation-prepared rapid gradient echo sequence to analyse brain volumetrics. Images will be subsequently analysed using standard procedures developed at the Knight ADRC using FreeSurfer to delineate brain regions,31 including cortical and subcortical areas, typically affected by AD.28
Functional MRI/network dysfunction
During the MRI scan, rs-fc scans will be obtained using a gradient spin-echo sequence. Participants will be instructed to fixate on a visual crosshair and not to fall asleep. Rs-fc pre-processing and post-processing will be performed using standardised, in-house methods.32 In preparation for correlation analysis, data will be spatially smoothed with a 6 mm full-width at half maximum Gaussian blur. Then, temporal low-pass filtering (f<0.1 Hz) will be applied to the time series of each voxel. Finally, spurious variance will be removed using linear regression for (1) six parameters generated from head motion correction, (2) the whole brain signal and (3) signals from ventricular and cerebral white matter. An ROI-based analysis consisting of 298 seeds will be performed with a Pearson’s correlation coefficient computed between pairwise ROI time courses across all areas within RSNs. From these 298 seeds, correlation matrices will be generated for each participant. For the 13 RSNs, correlation coefficients across ROI pairs within a network will be averaged to form a composite score. Based on average matrices, both intra-network (diagonal) and inter-network (off diagonal) composite scores will be generated.
CSF biomarkers
CSF will be collected at approximately 08:00 following overnight fasting.33 About 20–30 mL of CSF is collected, centrifuged briefly at low speed, aliquoted into polypropylene tubes and then stored at −80°C. Aβ40, Aβ42, total tau (tTau) and tau phosphorylated at 181 (pTau181) are measured by chemiluminescent enzyme immunoassay using a fully automated platform (LUMIPULSE G1200, Fujirebio)34 according to the manufacturer’s specifications. APOE genotype will be determined by genotyping rs7412 and rs429358 using Taqman genotyping technology as described previously.35
Preclinical AD staging
Biomarker positivity will be defined by correlating biomarker values at baseline with the risk of developing AD symptoms over time. The derivation of the biomarker cut-offs will be independent of the data collected in this project. Of note, CSF markers of tauopathy (pTau181) and neurodegeneration (tTau) are extremely highly correlated (r~0.96), so further stratification of stage by tauopathy would not be meaningful.36 Participants will be classified as follows: CN if measures of amyloid, neurodegeneration and episodic memory are normal; stage 1 if only measures of amyloid are abnormal by CSF Aβ42/Aβ40 or amyloid PET mean cortical SUVRs (which are highly concordant); stage 2 if only measures of amyloid and neurodegeneration (by CSF tTau) are abnormal; and stage 3 if measures of amyloid, neurodegeneration and episodic memory are abnormal.37
Annual in-home visit
An occupational therapist (OT), blinded to participants’ preclinical AD status, will complete a 120–180 min in-home visit annually for 4 years. The OT will conduct assessments related to the PNS as well as in-home functional mobility and recognised fall covariates (tables 1 and 2). Although the annual visit is typically completed in one session, it will be completed over two sessions if needed due to participant fatigue and/or request. Participants will receive a report with their results from the home visit and fall risk assessment based on established fall risk cut-off scores.38
Table 1.
Knight ADRC and in-home assessments
| Construct | Measure | Description | |
| Central nervous system | Attentional/executive control composite derived | Stroop colour naming task51* | Colour naming of congruent (eg, red), neutral (eg, deep) or incongruent (eg, blue) word |
| Simon task52* | Naming direction of an arrow with a keypress that is spatially consistent or inconsistent with the location of the arrow including congruent and incongruent positioning | ||
| Attentional switching task53* | Switching every other trial between making odd-even decisions and consonant-vowel decisions on bivalent stimuli (eg, B14) | ||
| Peripheral nervous system | Standing, balance and vestibular function | Centre of pressure path54 | Centre of pressure path will be measured using Balance Tracking System (BTrackS) |
| Lower extremity strength and function | 30-second chair stand test55 | A score below the norm will be considered indicative of decreased lower extremity strength and function | |
| Handheld dynamometer56 | Minimal change in the peak torque value for lower extremity strength will be measured | ||
| Grip strength | Handheld dynamometer57 | Pounds of force will be captured for grip strength | |
| Vision | Early Treatment Diabetic Retinopathy Study (ETDRS) test58 | Visual acuity score; number of correct letters read | |
| Pelli-Robson test59 | Contrast sensitivity; letter-by-letter | ||
| Sensation | Tuning fork, sharp60 | 8-Item questionnaire and sensation testing (vibration (feet) and sharp (arms and legs)) | |
| Functional mobility | Dynamic balance and mobility | Performance-Oriented Mobility Assessment (POMA)61 | A task-oriented assessment of 9 balance tasks and 7 items to assess gait |
| Gait speed | Timed Up and Go (TUG) test62 | Timed task of standing up, walking 3 m, turning, walking back and sitting down | |
| Dual-task gait | Timed Up and Go Cognitive (TUGcog)63 | TUG test while reciting serial 3s with subtractions from various points | |
| Dual-task gait | Timed Up and Go Manual (TUGman)64 | TUG test while carrying a glass of water | |
| Additional assessments | Alcohol abuse | Short Michigan Alcoholism Screening Test—Geriatric Version (SMAST-G)65 | 10-Item interview |
| Depression | Patient Health Questionnaire (PHQ-9)66 | 10-Item questionnaire to assess frequency of symptoms; 0–27 points | |
| Geriatric Depression Scale—Short Form (GDS-SF)67* | 15-Item questionnaire; 0–15 points | ||
| Urinary incontinence | Frequency and type68 | Short questionnaire of frequency and type (stress, urge or other) | |
| Pain | Self-report69 | Pain scale from 12-item Short Form Survey | |
| Medication | Medication review* | Medications and dosages | |
| Functional capacity | Older Adults Resources and Services Activities of Daily Living (OARS ADL) scale70 | Ability to perform 14 activities; 0–2 scale, higher scores indicate greater independence | |
| Functional performance | Performance Assessment of Self-Care Skills (PASS)71 | Evaluates independence, safety, and adequacy with shopping, chequebook balancing and medication management | |
| Falls behaviour | Falls Behavioural Scale for Older People (FaB)72 | 30-Item questionnaire; rated from 1 (least protective) to 4 (most protective) behaviours to prevent falls | |
| Self-efficacy | Falls Efficacy Scale—International (FES-ISF)73 |
7 daily activities; rated from 1 (not at all) to 4 (very concerned) about falling during specific activities | |
| Home hazards | Westmead Home Safety Assessment (WeSHA)74 | Rates 72 environmental home hazards as hazard/no hazard | |
| Olfaction | University of Pennsylvania Smell Identification Test (UPSIT)75 | 40-Item smell identification test; 0–40 points | |
| Hearing | Hearing Handicap Inventory for the Elderly Screening Version (HHIE-S)76 | 10-Item questionnaire to screen for hearing impairment; 0–40 points | |
| Brief Hearing Test | Screening tone test at varying frequencies |
*Collected at the Knight ADRC.
Knight ADRC, Knight Alzheimer Disease Research Center.
Table 2.
Fall covariate composite score variables
| Construct | Measure | Description | Fall risk cut-off38 |
| Vision | Early Treatment Diabetic Retinopathy Study (ETDRS) test58 | Visual acuity score; number of correct letters read | ≤12 |
| Pelli-Robson test59 | Contrast sensitivity; letter-by-letter | <36 letters | |
| Alcohol abuse | Short Michigan Alcoholism Screening Test—Geriatric Version (SMAST-G)65 | 10-Item interview | ≥2 |
| Depression | Geriatric Depression Scale—Short Form (GDS-SF)67* | 15-Item questionnaire; 0–15 points | >4 |
| Urinary incontinence | Frequency and type68 | Short questionnaire of frequency and type (stress, urge or other) | ≥weekly urge incontinence |
| Pain | Self-report69 | Pain scale from 12-item Short Form Survey | ≥moderate |
| Medication | Medication review* | Medications and dosages | ≥4 medications |
| Functional capacity | Older Adults Resources and Services Activities of Daily Living (OARS ADL) scale70 | Ability to perform 14 activities; 0–2 scale, higher scores indicate greater independence | >4 |
| Previous falls | Previous falls38 | Total falls in the past 12 months, self-report | >0 |
| Home hazards | Westmead Home Safety Assessment (WeSHA)74 | Rates 72 environmental home hazards as hazard/no hazard | ≥4 hazards |
| Self-efficacy | Falls Efficacy Scale—International (FES-ISF)73 |
7 daily activities; rated from 1 (not at all) to 4 (very concerned) about falling during specific activities | >10 |
*Collected at the Knight ADRC.
Monthly fall reporting
Participants will report falls prospectively via automated call or email every month for 4 years using the gold standard for fall reporting, including daily calendar journals, fall interviews and monetary compensation for reporting.39 Participants will also receive a standardised fall report form to record the time and location of a fall, nature of the fall environment, specific activity at the time of the fall and any somatic complaints that proceeded the fall.40 If a participant reports a fall, an interviewer blinded to preclinical AD status will call the participant to complete a fall interview to verify the fall, defined as an unintentional movement to the floor, ground or an object below knee level. The interviewer will then gather additional information about any subsequent injuries or medical treatment.9 41 42 The rate (number) and severity (calculated with a standardised algorithm from medical records and participant report) of falls will be generated.13 The falls severity score will be quantified using a previously published algorithm: no falls (0), 1 fall without serious injury (1), any fall with minor injury or more than 1 fall (2), and major injury requiring hospitalisation (3).14
Measures
An overview of the assessments collected at the Knight ADRC and annual in-home visits, including CNS and PNS measures, functional mobility, additional covariates of interest and fall covariates, for this study are listed in tables 1 and 2.
Statistical analysis plan
Data will be entered into Research Electronic Data Capture (REDCap),43 a secure, web-based application, and analysed using SAS, V.9.4 (SAS Institute). Differences in baseline characteristics across groups will be compared using appropriate statistics (χ2 test, Student’s t-test or Mann-Whitney U test). Composites and cut-offs will be calculated as described in the Methods and analysis section (see table 2). Models for analysing AD biomarkers and cognition will include age, gender, fall risk composite score, APOE status (at least APOE ε4 allele), as well as possible interactions among study variables. Models will be implemented using PROC GLM or PROC MIXED/SAS.
Statistical analysis plan for the primary aim
We will examine the distributions of falls (number and severity) over a 1-year follow-up window and baseline functional mobility scores across the preclinical stages of AD (0, 1, 2 and 3),4 with appropriate transformations as needed. Falls severity scores across preclinical stages will be compared using analysis of covariance models.44 Similar analyses will be conducted to compare each of the functional mobility measures across the preclinical stages of AD. We will implement adequate approaches (eg, Benjamini-Hochberg false discovery procedure)45 to control for the overall type I error rate due to multiple outcome variables (number and severity of falls, functional mobility) tested in this aim.
We will also jointly model the longitudinal falls severity score and the time-to-symptom onset of AD (defined as the first time a participant receives a CDR >0) using general linear mixed effects models.46 For modelling the risk of developing AD, we will use the semiparametric Cox proportional hazards model. To address the association between change in falls and the risk of developing symptomatic AD, we will implement joint models.47 48
Statistical analysis plan for the secondary aim
We will test a hypothesised model of attentional compared with perceptual/motor systems underlying falls in preclinical AD using structural equation models (SEMs) on cross-sectional data.49 The structural model will include the estimation of path coefficients among various latent constructs including brain neuropathology, network dysfunction, PNS abnormalities and falls. We will fit and compare various SEMs for their goodness-of-fit through standard statistics using multiple models.
Sample size calculations
Primary aim
To examine the relationship between falls, functional mobility and AD, we will enrol 350 older adults from the Knight ADRC. Based on the distribution of CN participants across clinical stages in the existing Knight ADRC database, the proposed sample size will provide at least 80% statistical power to detect an effect size as small as 0.225 SD on the falls severity score between two adjacent participant groups. From the Knight ADRC database, we fitted a survival curve from baseline to the time that a CDR >0 was first rendered. We found an estimated CDR progression rate of 7.2% per year for individuals with a mean age of 75 at baseline and an expected attrition of approximately 15%. We estimate that approximately 300 participants will be assessed annually throughout the study, and approximately 75 of these individuals will progress to CDR >0 after baseline. This will provide at least 80% statistical power to detect a onefold increase in the risk of developing symptomatic AD for individuals with an increased rate of falls over time compared with those with slow or no changes in falls over time. These power computations were based on a log rank test at the 5% significance level and assumed an annual rate of 4.7% of CDR progression for individuals with slow changes in disability over time.
Secondary aim
We also tested non-zero path coefficients that link the latent constructs of network dysfunction with attentional compared with perceptual/motor systems, and to impaired functional mobility and falls. The proposed sample provides at least 80% statistical power to detect each path coefficient.
Participants and public involvement
Participants and the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Ethics and dissemination
This study was approved by the Washington University in St. Louis Institutional Review Board (reference number 201807135). Written informed consent will be obtained in the home prior to the collection of any study data. Participants may withdraw from the study at any time. Results will be published in peer-reviewed publications and presented at national and international conferences.
Discussion
Changes in functional mobility and an increase in falls may be an early indicator of preclinical AD.3 16 50 Underlying deviations in functional connectivity may assist in identifying brain RSNs that are affected and lead to falls.17 Measures of everyday function are not currently included in the evaluation of CN individuals with preclinical AD. To examine these relationships, this study will assess the number and severity of falls, functional mobility (gait and balance), and changes in functional connections (rs-fc) within and across RSNs in a sample of community-dwelling older adults. This will allow us to characterise when changes in falls and functional mobility occur during the preclinical stages of AD as well as potential mechanisms.
The strengths of this study include access to a large, well-characterised cohort of community-dwelling older adults at the Knight ADRC who are enthusiastic about participating in studies. Another strength includes a comprehensive in-home evaluation of a participant’s fall risks and functional mobility and the ability to share results with each participant.
Although the strengths are promising, there are a few limitations to this study. First, older adults may not be compliant with fall monitoring over time. The OTs will call participants to obtain fall information if participants do not want to complete the fall monitoring via automated call or email. Lastly, it may be difficult to differentiate falling from ageing-related phenotypes such as frailty. We will collect information on covariates, including comorbid conditions and other fall risk factors, test these relationships in individuals without preclinical AD and control for these covariates in statistical analyses.
This study is designed to examine the relationship between falls and functional mobility and underlying attentional compared with perceptual/motor systems in preclinical stages of AD. The findings will enhance our understanding of the systemic manifestations of AD and may identify falls as a previously unknown risk factor for developing preclinical AD. If successful, this study can potentially inform the timing of interventions in secondary prevention trials in AD as well as the development of more precise, effective treatments for individuals at risk for progression to symptomatic AD.18
Supplementary Material
Acknowledgments
We would like to thank the Knight Alzheimer Disease Research Center staff and all of our participants for making this study possible.
Footnotes
Twitter: @PEPLaboratory
Contributors: The study concept and design was conceived by SLS, BMA, CX, DB and JCM. Patient safety protocols and IRB compliance will be managed by EW, RT and RMB. Recruitment and data collection will be conducted by RMB, RT and AK. Analysis will be performed by AMF, TLSB, SES and CX. The first draft of the protocol was prepared by BMA and SLS. All authors provided edits and approved the final version of the protocol.
Funding: This work was supported by the National Institute on Aging (1 R01 AG057680-01A1), the Healthy Aging and Senile Dementia Program Project (P01 AG03991), the Knight Alzheimer Disease Research Center (P30 AG066444), Antecedent Biomarkers for AD: the Adult Children Study (P01 AG026276), the Paula C. and Rodger O. Riney Fund, and the Daniel J. Brennan MD Fund.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.World Health Organization . Fact sheets: dementia: World Health organization, 2020. Available: https://www.who.int/en/news-room/fact-sheets/detail/dementia2020 [Accessed 21 Sep 2020].
- 2.Patterson C. World Alzheimer report 2018: the state of the art of dementia research: new frontiers. London: Alzheimer Disease International, 2018. [Google Scholar]
- 3.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–31. 10.1002/ana.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–92. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012;367:795–804. 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–65. 10.1016/S1474-4422(13)70194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–47. 10.1212/WNL.0000000000002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggenberger P, Wolf M, Schumann M, et al. Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front Aging Neurosci 2016;8:66. 10.3389/fnagi.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark SL, Roe CM, Grant EA, et al. Preclinical Alzheimer disease and risk of falls. Neurology 2013;81:437–43. 10.1212/WNL.0b013e31829d8599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montero-Odasso M, Oteng-Amoako A, Speechley M, et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci 2014;69:1415–21. 10.1093/gerona/glu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement 2015;11:70–98. 10.1016/j.jalz.2014.04.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer's disease. Dement Geriatr Cogn Disord 2007;24:125–37. 10.1159/000105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–7. 10.1056/NEJM198812293192604 [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med Overseas Ed 1997;337:1279–84. 10.1056/NEJM199710303371806 [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Rubin EH, Morris EJ, et al. Senile dementia of the Alzheimer's type: an important risk factor for serious falls. J Gerontol 1987;42:412–7. 10.1093/geronj/42.4.412 [DOI] [PubMed] [Google Scholar]
- 16.McGough EL, Lin S-Y, Belza B, et al. A scoping review of physical performance outcome measures used in exercise interventions for older adults with Alzheimer disease and related dementias. J Geriatr Phys Ther 2019;42:28–47. 10.1519/JPT.0000000000000159 [DOI] [PubMed] [Google Scholar]
- 17.Brier MR, Thomas JB, Snyder AZ, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 2012;32:8890–9. 10.1523/JNEUROSCI.5698-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansai JH, Andrade LPde, Masse FAA, et al. Risk factors for falls in older adults with mild cognitive impairment and mild Alzheimer disease. J Geriatr Phys Ther 2019;42:E116–21. 10.1519/JPT.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 20.Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol 2019;76:264–73. 10.1001/jamaneurol.2018.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg L, McKeel DW, Miller JP, et al. Neuropathological indexes of Alzheimer's disease in demented and nondemented persons aged 80 years and older. Arch Neurol 1993;50:349–58. 10.1001/archneur.1993.00540040011008 [DOI] [PubMed] [Google Scholar]
- 22.Storandt M, Hill RD. Very mild senile dementia of the Alzheimer type. II. psychometric test performance. Arch Neurol 1989;46:383–6. 10.1001/archneur.1989.00520400037017 [DOI] [PubMed] [Google Scholar]
- 23.Duchek JM, Balota DA, Thomas JB, et al. Relationship between Stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology 2013;27:516–28. 10.1037/a0033402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aschenbrenner AJ, Balota DA, Fagan AM, et al. Alzheimer disease cerebrospinal fluid biomarkers moderate baseline differences and predict longitudinal change in attentional control and episodic memory composites in the adult children study. J Int Neuropsychol Soc 2015;21:573–83. 10.1017/S1355617715000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschenbrenner AJ, Balota DA, Tse C-S, et al. Alzheimer disease biomarkers, attentional control, and semantic memory retrieval: synergistic and mediational effects of biomarkers on a sensitive cognitive measure in non-demented older adults. Neuropsychology 2015;29:368–81. 10.1037/neu0000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Y, D'Angelo GM, Vlassenko AG, et al. Quantitative analysis of PIB-PET with FreeSurfer ROIs. PLoS One 2013;8:e73377. 10.1371/journal.pone.0073377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med 2012;53:378–84. 10.2967/jnumed.111.090340 [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Benzinger TL, Hassenstab J, et al. Spatially distinct atrophy is linked to β-amyloid and tau in preclinical Alzheimer disease. Neurology 2015;84:1254–60. 10.1212/WNL.0000000000001401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrillo MC, Blackwell A, Hampel H, et al. Early risk assessment for Alzheimer's disease. Alzheimers Dement 2009;5:182–96. 10.1016/j.jalz.2009.01.019 [DOI] [PubMed] [Google Scholar]
- 30.Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer's disease. Neurobiol Dis 2009;35:128–40. 10.1016/j.nbd.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- 32.Shulman GL, Pope DLW, Astafiev SV, et al. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 2010;30:3640–51. 10.1523/JNEUROSCI.4085-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–9. 10.1002/ana.20730 [DOI] [PubMed] [Google Scholar]
- 34.Kaplow J, Vandijck M, Gray J, et al. Concordance of Lumipulse cerebrospinal fluid t-tau/Aβ42 ratio with amyloid PET status. Alzheimers Dement 2020;16:144–52. 10.1002/alz.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruchaga C, Kauwe JSK, Mayo K, et al. Snps associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer's disease. PLoS Genet 2010;6:e1001101. 10.1371/journal.pgen.1001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soldan A, Pettigrew C, Fagan AM, et al. ATN profiles among cognitively normal individuals and longitudinal cognitive outcomes. Neurology 2019;92:e1567–79. 10.1212/WNL.0000000000007248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra S, Gordon BA, Su Y, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage 2017;161:171–8. 10.1016/j.neuroimage.2017.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lusardi MM, Fritz S, Middleton A, et al. Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. J Geriatr Phys Ther 2017;40:1–36. 10.1519/JPT.0000000000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamb SE, Jørstad-Stein EC, Hauer K, et al. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc 2005;53:1618–22. 10.1111/j.1532-5415.2005.53455.x [DOI] [PubMed] [Google Scholar]
- 40.Lach HW, Reed AT, Arfken CL, et al. Falls in the elderly: reliability of a classification system. J Am Geriatr Soc 1991;39:197–202. 10.1111/j.1532-5415.1991.tb01626.x [DOI] [PubMed] [Google Scholar]
- 41.Stark SL, Silianoff TJ, Kim HL, et al. Tailored calendar journals to ascertain falls among older adults. OTJR 2015;35:53–9. 10.1177/1539449214561764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark S, Somerville E, Conte J, Keglovits M, et al. Feasibility trial of tailored home modifications: process outcomes. Am J Occup Ther 2018;72:7201205020p1–20. 10.5014/ajot.2018.021774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milliken GA, Johnson DE, eds. Analysis of Messy Data. New York: Chapman & Hall/CRC, 2001. [Google Scholar]
- 45.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 46.Diggle P, Liang K-Y, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press, 1994. [Google Scholar]
- 47.Jacqmin-Gadda H, Commenges D, Dartigues J-F. Random change point model for joint modeling of cognitive decline and dementia. Biometrics 2006;62:254–60. 10.1111/j.1541-0420.2005.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Gruttola V, Tu XM. Modelling progression of CD4-lymphocyte count and its relationship to survival time. Biometrics 1994;50:1003–14. 10.2307/2533439 [DOI] [PubMed] [Google Scholar]
- 49.Kline RB. Principles and practice of structural equation modeling. Fourth ed. New York: The Guilford Press, 2015. [Google Scholar]
- 50.Stark SL, Roe C, Grant E. Risk of falls in older adults with preclinical Alzheimer’s disease. Paris, France: Alzheimer’s Association International Conference on Alzheimer’s Disease, 2011. [Google Scholar]
- 51.MacLeod CM. The Stroop task: The "gold standard" of attentional measures. J Exp Psychol 1992;121:12–14. 10.1037/0096-3445.121.1.12 [DOI] [Google Scholar]
- 52.Simon JR. Reactions toward the source of stimulation. J Exp Psychol 1969;81:174–6. 10.1037/h0027448 [DOI] [PubMed] [Google Scholar]
- 53.Schmitter-Edgecombe M, Langill M. Costs of a predictable switch between simple cognitive tasks following severe closed-head injury. Neuropsychology 2006;20:675–84. 10.1037/0894-4105.20.6.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Connor SM, Baweja HS, Goble DJ. Validating the BTrackS balance plate as a low cost alternative for the measurement of sway-induced center of pressure. J Biomech 2016;49:4142–5. 10.1016/j.jbiomech.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 55.Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults, ages 60-94. J Aging Phys Act 1999;7:162–81. 10.1123/japa.7.2.162 [DOI] [Google Scholar]
- 56.O'Shea SD, Taylor NF, Paratz JD. Measuring muscle strength for people with chronic obstructive pulmonary disease: retest reliability of hand-held dynamometry. Arch Phys Med Rehabil 2007;88:32–6. 10.1016/j.apmr.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 57.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 2008;31:3–10. 10.1519/00139143-200831010-00002 [DOI] [PubMed] [Google Scholar]
- 58.Ferris FL, Kassoff A, Bresnick GH, et al. New visual acuity charts for clinical research. Am J Ophthalmol 1982;94:91–6. 10.1016/0002-9394(82)90197-0 [DOI] [PubMed] [Google Scholar]
- 59.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci 1988;2:187–99. [Google Scholar]
- 60.Kästenbauer T, Sauseng S, Brath H, et al. The value of the Rydel-Seiffer tuning fork as a predictor of diabetic polyneuropathy compared with a neurothesiometer. Diabet Med 2004;21:563–7. 10.1111/j.1464-5491.2004.01205.x [DOI] [PubMed] [Google Scholar]
- 61.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986;34:119–26. 10.1111/j.1532-5415.1986.tb05480.x [DOI] [PubMed] [Google Scholar]
- 62.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 63.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896–903. 10.1093/ptj/80.9.896 [DOI] [PubMed] [Google Scholar]
- 64.Lundin-Olsson L, Nyberg L, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc 1998;46:758–61. 10.1111/j.1532-5415.1998.tb03813.x [DOI] [PubMed] [Google Scholar]
- 65.Blow FC, Brower KJ, Schulenberg JE. The Michigan alcoholism screening test – geriatric version (MAST-G): a new elderly-specific screening instrument. Alcohol Clin Exp Res 1992;16:372. [Google Scholar]
- 66.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA 1999;282:1737–44. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 67.Sheikh J, Yesavage J. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986;5:165–73. [Google Scholar]
- 68.Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? J Am Geriatr Soc 2000;48:721–5. 10.1111/j.1532-5415.2000.tb04744.x [DOI] [PubMed] [Google Scholar]
- 69.Gandek B, Ware JE, Aaronson NK, et al. Cross-Validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol 1998;51:1171–8. 10.1016/s0895-4356(98)00109-7 [DOI] [PubMed] [Google Scholar]
- 70.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 1981;36:428–34. 10.1093/geronj/36.4.428 [DOI] [PubMed] [Google Scholar]
- 71.Rogers J, Holm M. Performance assessment of self-care skills. unpublished performance test. Pittsburgh, PA: University of Pittsburgh, 1989. [Google Scholar]
- 72.Clemson L, Cumming RG, Heard R. The development of an assessment to evaluate behavioral factors associated with falling. Am J Occup Ther 2003;57:380–8. 10.5014/ajot.57.4.380 [DOI] [PubMed] [Google Scholar]
- 73.Kempen GIJM, Yardley L, van Haastregt JCM, et al. The short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing 2008;37:45–50. 10.1093/ageing/afm157 [DOI] [PubMed] [Google Scholar]
- 74.Clemson L, Roland M, Cumming RG. Types of hazards in the homes of elderly people. Occup Ther J Res 1997;17:200–13. 10.1177/153944929701700304 [DOI] [Google Scholar]
- 75.Doty RL, Shaman P, Kimmelman CP, et al. University of Pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984;94:176–8. 10.1288/00005537-198402000-00004 [DOI] [PubMed] [Google Scholar]
- 76.Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA 1983;25:37–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


