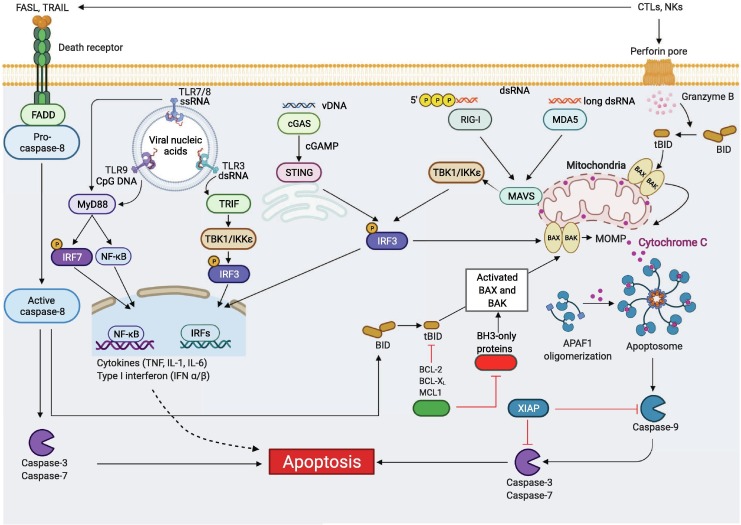
Figure 2.
Molecular mechanisms of apoptosis activation during viral infection. Extrinsic apoptosis is activated through engagement of death receptors by their cognate ligands. One example is the activation of FAS by FASL expressed on cytotoxic T lymphocytes (CTLs) or natural killer (NK) cells. The adaptor protein FADD together with pro–caspase-8 are then recruited to the death receptor’s cytosolic domain, inducing caspase-8 activation. Active caspase-8 directly induces proteolytic cleavage of the executioner caspases, caspase-3 and -7, triggering extrinsic apoptosis. Active caspase-8 also can cleave the BH3-only protein BID to form tBID, inducing intrinsic apoptosis. Intrinsic apoptosis is triggered by internal cellular stress such as DNA damage or ER stress, inducing the activation of pro-apoptotic BH3-only proteins including NOXA, PUMA, BAD and BIM. This activation triggers BAX and BAK activation to induce mitochondrial outer membrane permeabilization (MOMP). MOMP leads to the release of apoptogenic factors including cytochrome C, which binds to apoptotic peptidase activating factor 1 (APAF1) and induces APAF1 oligomerization to form the apoptosome. Apoptosome formation activates the initiator caspase-9, which then cleaves executioner caspases, caspase-3 and -7 to drive intrinsic apoptosis. Intrinsic apoptosis can also be activated through active caspase-8 or in response to the release of protease granzyme B through the perforin pore initiated by CTLs and NK cells, mediating the cleavage of BID to its active form tBID, triggering BAX-BAK to induce MOMP and apoptosis. Furthermore, recognition of viral nucleic acids through TLRs in the endosome or cytosolic PRRs, such as cGAS-STING (vDNA), RIG-I (triphosphates-vRNA), and MDA5 (long vdsRNA), can trigger pro-apoptotic and inflammatory transcription factor NF-κB and type I IFN production, which can induce pro-apoptotic gene expression and subsequent apoptosis. Created with BioRender.com.