Figure 1.
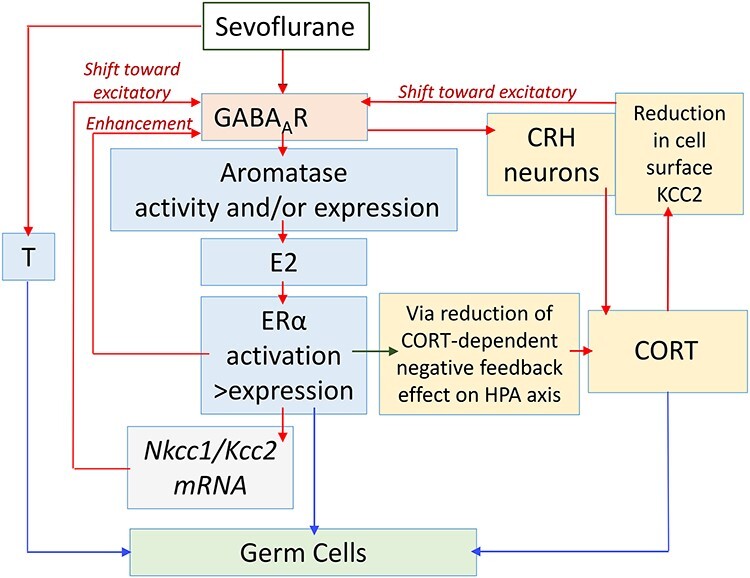
A diagram illustrating hypothetical pathways that involve the positive feedback interaction between GABAAR signaling, stress, and sex steroids in the mediation of heritable effects of sevoflurane. (See text for details.) These hypothetical pathways are proposed based on our published experimental findings and/or data in the literature that are discussed in this minireview; nevertheless, these are hypotheses that require rigorous experimental verification. The red arrows illustrate positive, stimulatory effects and the green arrow illustrates a negative, inhibitory effect. The blue arrows illustrate effects of steroids on parental germ cells. Our experimental data indicate that sevoflurane acts via GABAAR-independent mechanisms to increase systemic levels of testosterone (T) in males only [69]. Sevoflurane, by acting via GABAAR-mediated mechanisms, increases systemic levels of 17-β-estradiol (E2) in males and females [68, 69]. Our findings show that sevoflurane increases expressions of aromatase and estrogen receptor α (Erα), but not estrogen receptor β (Erβ), and systemic E2 levels. E2, by acting via ERα, increases the Na+-K+-Cl− cotransporter (Nkcc1)/K+-Cl− cotransporter (Kcc2) ratio. Therefore, E2 may shift GABAAR signaling toward excitatory and further enhances GABAAR excitatory signaling through direct interaction with GABAAR, as evident from increased sevoflurane-induced electroencephalography (EEG)-detectable seizure-like activities [68, 69]. The enhanced excitatory GABAAR signaling leads to a further increase in sevoflurane-stimulated production of E2. Sevoflurane, by enhancing excitatory GABAAR signaling in corticotrophin-releasing hormone (CRH) neurons in the paraventricular nucleus (PVN) of the hypothalamus, stimulates the hypothalamic–pituitary–adrenal (HPA) axis and corticosterone (CORT) production [59, 67–69]. The stress response reduces the KCC2 cell-surface expression in the hypothalamic neurons [59], leading to a shift in GABAAR signaling toward excitatory, and, as a result, to greater upregulation of the HPA and hypothalamic-pituitary-gonadal (HPG) axes. Sevoflurane, by increasing E2 levels and expression of Erα, reduces the CORT-dependent negative feedback on the HPA axis [101, 102], leading to greater stress response, more excitatory GABAAR signaling via reduced cell surface KCC2 expression, and greater GABAAR signaling-mediated production of E2. Based on data in the literature on epigenetic effects of stress in germ cells [138, 140–143] and on our own findings [58, 59], we hypothesize that CORT mediates effects of sevoflurane in parental germ cells, at least in part through epigenetic modifications. The heritable effects of EDCs, as well as transcriptomic activities of ERα and androgen receptors (AR) [9, 15, 136, 137, 145–147], allow us to hypothesize that E2 and T may also mediate heritable effects of sevoflurane through their actions in parental germ cells.
