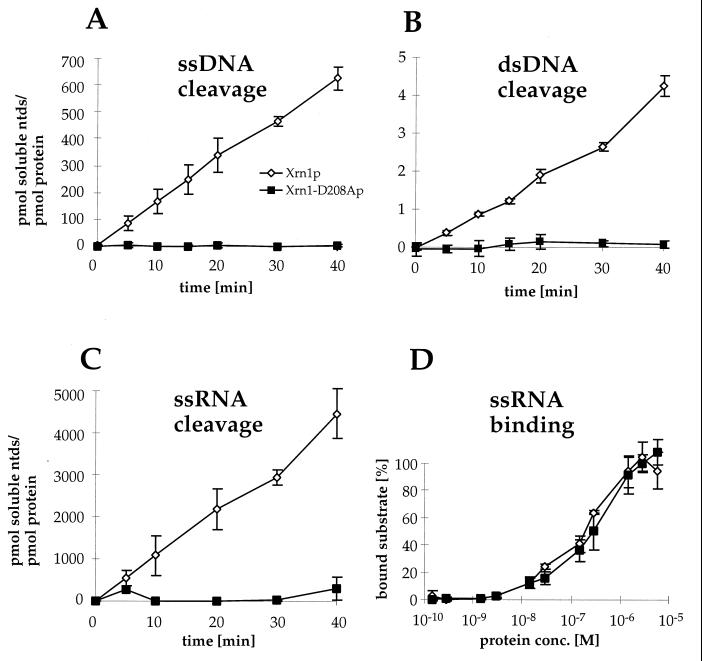
FIG. 6.
In vitro exonuclease activity of Xrn1-D208A protein is abolished, but substrate binding is retained. (A to C) Time course reactions with Xrn1p and Xrn1-D208Ap were performed on homogeneously labeled substrates: nuclease assay with HaeIII digested bacteriophage T7 ssDNA (A), nuclease assay with HaeIII digested bacteriophage T7 dsDNA (B), and nuclease assay with ssRNA transcript from the mouse actin gene (C). (D) Binding of Xrn1p and Xrn1-D208Ap to actin RNA (nuclease substrate of panel C) was determined by filter binding. Each graph represents the mean and standard deviations of three experiments.