Figure 3. Recombinant human diamine oxidase (rhDAO) heparin-binding motif (HBM) double mutants show significantly reduced uptake into various cell lines.
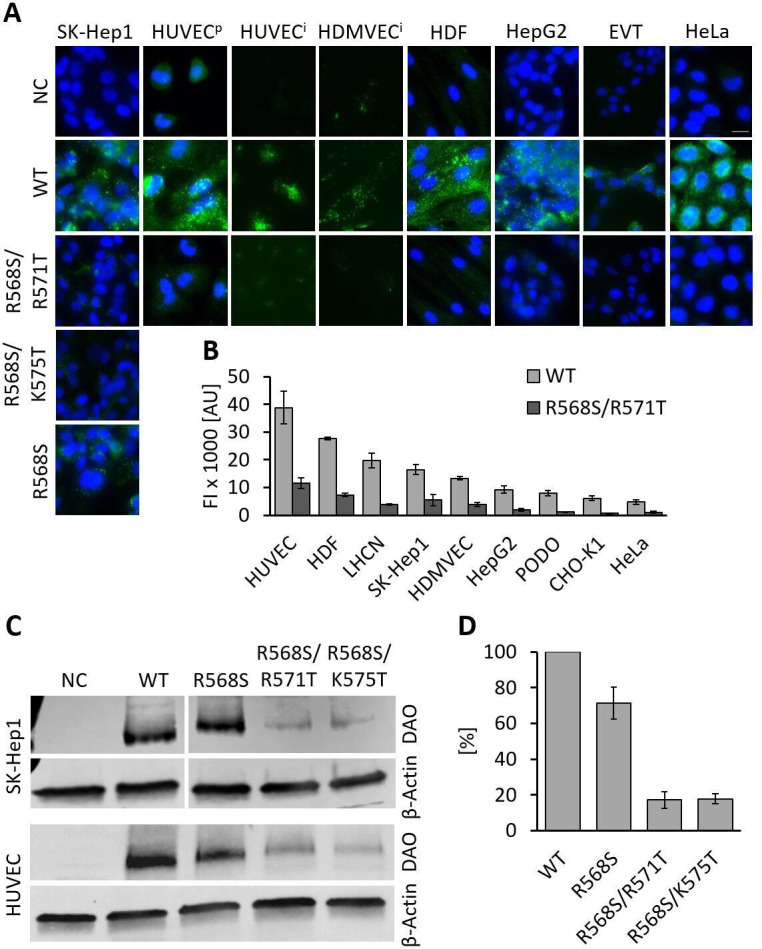
(A) SK-Hep1, primary human umbilical vein endothelial cells ( = HUVECp), human dermal fibroblasts (HDF), HepG2, and HeLa cells were incubated with 120 nM unlabeled purified rhDAO-WT and various HBM mutants and detected with rabbit anti-ABP1 antibodies and Alexa Fluor 488 donkey anti-rabbit antibodies after fixation and permeabilization. Immortalized HUVEC/TERT2 ( = HUVECi) and human dermal microvascular endothelial cells (HDMVEC/TERT164-B = HDMVECi) were incubated with 120 nM and extravillous trophoblasts (EVT) with 60 nM Alexa488-labeled rhDAO-WT and rhDAO-R568S/R571T; Scale bar = 20 μm. (B) Cells were incubated with 30 nM Alexa488-labeled rhDAO-WT and rhDAO-R568S/R571T mutant (no DAO added = negative control), washed, and analyzed flow cytometrically (500 cells per sample). Background corrected mean median values ± standard error of the mean (SEM) are shown (n = 4 biological replicates, two individual experiments in duplicate). (C) 106 SK-Hep1 and 5 × 105 HUVEC/TERT2 cells were incubated with 120 nM and 60 nM rhDAO-WT and three HBM mutants. The cell lysates were analyzed for DAO uptake using western blotting. β-Actin was used as an internal standard. (D) After background subtraction, rhDAO band intensities were corrected against β-Actin and normalized against the rhDAO-WT band. The mean ± SEM of two individual experiments with SK-Hep1 cells and one experiment with HUVEC/TERT2 cells are shown. (A–D) Incubations were performed for 60 min at 37°C. FI: fluorescence intensity; AU: arbitrary units.
