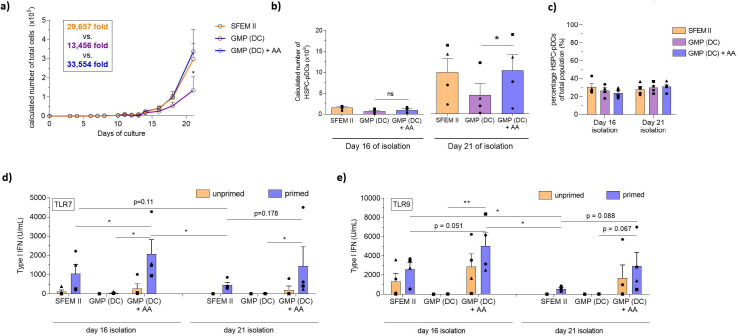
Figure 4. Ascorbic acid medium supplementation is required for hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cell (HSPC-pDC) generation using the current good manufacturing process (cGMP)-compliant DC medium.
HSPCs were thawed and 1 × 105 cells were seeded in SFEM II, the cGMP-compliant medium DC medium (GMP [DC]), or DC medium supplemented with ascorbic acid (GMP [DC] + AA). For all conditions, cells were kept at a density of 0.5–5 × 106 cells/mL throughout culture. HSPC-pDCs were isolated after 16 and 21 days of culture and phenotypically analyzed. (a) Calculated number of total cells during HSPC-pDC differentiation. (b) Calculated number of HSPC-pDCs after isolation at 16 days or 21 days of culture. (c) Percentage of HSPC-pDCs of total cells. (d, e) Type I interferon (IFN) response of IFN primed or unprimed HSPC-pDCs isolated after 16 or 21 days of culture after activation with the TLR7 agonist R837 (d) or the TLR9 agonist CpG-2216 (e). Data shown represent ± SEM of four donors (a–c) and ± SEM of four donors each analyzed in technical triplicates (d, e).