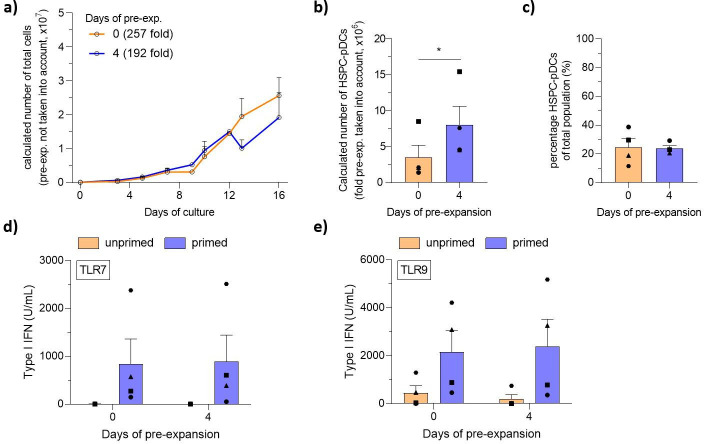
Figure 6. Generation of hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cells from circulating HSPCs (cHSPCs) from peripheral whole blood using optimized current good manufacturing process (cGMP)-compliant medium.
cHSPCs were pre-expanded for 4 days at low density (1–5 × 105 cells/mL) in cGMP-compliant medium (SCGM) supplemented with UM171 and then cryopreserved. Subsequently, cells were thawed, phenotyped for CD34, and 1 × 105 cHSPCs were seeded for HSPC-pDC generation. HSPC-pDCs were isolated after 16 days of culture and phenotypically analyzed. (a) Calculated number of cells during HSPC-pDC differentiation using pre-expanded HSPCs (without the pre-expansion factor taken into account). (b) Calculated number of HSPC-pDCs upon isolation of HSPC-pDCs at 16 days of culture (with fold pre-expansion taken into account). (c) Percentage of HSPC-pDCs of the total population of cells. (d, e) Levels of type I IFN upon stimulation of HSPC-pDCs with the TLR7 agonist R837 (d) or the TLR9 agonist CpG-2216 (e). Data shown represent ± SEM of four donors (a–c) and four donors each analyzed in technical triplicates (d, e).