Abstract
Background. Aging-associated noncommunicable comorbidities are more prevalent among human immunodeficiency virus type 1 (HIV)–infected individuals than among HIV-uninfected individuals. Residual HIV-related chronic immune activation and senescence may increase the risk of developing comorbidities.
Methods. Immune phenotyping, thymic output, and telomere length were assessed in 94 HIV-infected individuals who were aged >45 years and receiving antiretroviral therapy (ART; cases) and 95 age-matched uninfected controls.
Results. Cases had lower CD4+ T-cell counts, higher CD8+ T-cell counts, and increased levels of immune activation (ie, increased soluble CD14 [sCD14] level and increased percentages of CD38+HLA-DR+ cells among both CD4+ and CD8+ T cells), regulatory T cells, and percentage of programmed cell death 1 (PD-1)–expressing cells among CD4+ T cells. Immune senescence levels (ie, percentages of CD27−CD28− cells or CD57+ cells) were comparable between cases and controls. Peripheral blood mononuclear cells from cases had shorter telomeres but increased single-joint T-cell receptor excision circle content and CD31+ naive CD4+ T cells. Although cytomegalovirus (CMV) antibody titers were higher in cases, CMV-specific T-cell responses were comparable between cases and controls. T-cell senescence in cases was independently associated with T-cell activation but not with CMV-specific immune responses.
Conclusions. Despite long-term receipt of ART, HIV-infected adults had higher levels of immune activation, regulatory T cells, and PD-1–expressing CD4+ cells and shorter telomeres. The increased soluble CD14 levels and percentage of CD38+HLA-DR+ cells among CD4+ T cells correlated with shorter telomeres and increased regulatory T-cell levels. This suggests that HIV influences immune function irreversibly, with several pathways that are persistently abnormal during effective ART. Therapies aimed at improving immune health during ART are needed.
Keywords: immune activation, senescence, HIV, ART, thymic output, telomeres
The introduction of combination antiretroviral therapy (ART) has resulted in a dramatic decline in human immunodeficiency virus (HIV)–associated morbidity and mortality [1, 2]. However, ART does not fully restore health, and the life expectancy of HIV-infected ART recipients remains ≥10 years shorter than for the general population of the same age [2]. HIV-infected ART recipients are at higher risk for age-associated non–AIDS-related morbidity and mortality [3–5], and it has been hypothesized that HIV may influence aging [4]. Several factors have been implicated in contributing to this excess morbidity, including ART toxicity and chronic immune activation and dysfunction [6, 7].
In the general population, aging and age-associated diseases have been related to chronic inflammation and changes in innate and adaptive immunity [6]. During natural aging, low-grade chronic inflammation is characterized by elevated levels of inflammatory biomarkers such as interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and C-reactive protein [8]. Moreover, several immune parameters have been associated with age, including a shift in naive T cells toward memory cells, decreased thymic output, increased numbers of T cells lacking CD28, increased levels of activation markers (CD38/HLA-DR), poor proliferative ability (eg, programmed cell death 1 [PD-1] and CD57 expression), and extensive proliferative history (eg, CD57 expression and shorter telomeres) [9]. These age-associated changes in adaptive immunity are generally referred to as “immune senescence.” Chronic viral infections such as HIV infection and cytomegalovirus (CMV) infection are considered major contributors toward inducing immune senescence [9–11].
Chronic immune activation and inflammation are hallmarks of untreated HIV infection and, to a lesser degree, treated HIV infection [7, 12]. Several factors have been implicated in contributing to chronic immune activation and inflammation in HIV infection [7, 12]. HIV infects activated CCR5+CD4+ T cells, and depletion of these cells in gut-associated lymphoid tissue results in disruption of the intestinal barrier and increased translocation of microbial products. Ongoing HIV production and perhaps even spread/replication may allow continued exposure of the immune system to viral antigens, even during effective ART. Persistent immune dysfunction as a result of CD4+ T-cell depletion and incomplete immune recovery during ART may contribute to poor clearance of other viruses (eg, hepatitis C virus) or reactivation of viruses (eg, CMV), providing additional antigenic stimulation of the immune system. Persistent HIV-related inflammation and chronic immune activation may therefore accelerate T-cell proliferation and differentiation, eventually overwhelming the restorative capacity of the system [7, 12]. Indeed, even during ART, HIV-infected individuals have increased frequencies of senescent CD8+ T cells [6, 9, 11, 13]. Furthermore, the effect of CMV infection and increasing age on immune senescence is thought to be amplified by HIV infection [9].
The AGEhIV Cohort Study was implemented in 2010 in Amsterdam, the Netherlands, to compare the prevalence, incidence, and risk factors of aging-associated noncommunicable comorbidities and organ dysfunction among HIV-infected individuals and HIV-uninfected controls [5]. Most previous comparisons of individuals with and those without HIV disease were unable to fully adjust for several confounding cofactors, including age, ethnicity, lifestyle, and demographic characteristics. To address this concern, HIV-uninfected participants were recruited from the sexual health clinic of the Amsterdam Public Health Service or among uninfected participants in the existing Amsterdam Cohort studies on HIV/AIDS. We regularly monitored the distribution of age, sex, and ethnicity in both study groups and adjusted enrollment of underrepresented categories among HIV-uninfected participants accordingly. From this cohort, we selected HIV-infected adults who were older than 45 years, were receiving effective ART, and had no detectable viremia (<50 copies/mL) for at least 12 months. We selected HIV-uninfected adults who were also >45 years old. Immune parameters indicative of chronic immune activation and immune senescence were analyzed and compared between HIV-infected individuals with suppressed viremia during ART and controls. Factors independently contributing to immune activation and immune senescence were identified.
METHODS
Subjects
For this study, 95 HIV-infected individuals who had an undetectable plasma HIV RNA level (<50 copies/mL) for >12 months and 94 HIV-uninfected controls were randomly selected from the AGEhIV Cohort Study. The AGEhIV Cohort Study was implemented to compare prevalence, incidence, and risk factors of comorbidities and organ dysfunction among HIV-infected individuals and HIV-uninfected controls. HIV-infected participants were recruited from the HIV outpatient clinic of the Academic Medical Center in Amsterdam, and HIV-uninfected participants (controls) were recruited from the sexual health clinic of the Amsterdam Public Health Service or among uninfected participants in the existing Amsterdam Cohort Studies on HIV/AIDS (available at: http://www.amsterdamcohortstudies.org). Participants were aged ≥45 years with laboratory-confirmed presence or absence of HIV infection. For this particular substudy, we regularly monitored the distribution of age among the HIV-infected individuals and the HIV-uninfected controls and adjusted enrollment on the basis of underrepresentation of certain age groups accordingly. All analyses were performed data from the baseline time point.
CMV-specific T-cell responses were analyzed in a selection of participants from whom sufficient viable frozen peripheral blood mononuclear cells (PBMCs) were available. HIV-infected individuals and HIV-uninfected controls were matched for age: the mean age (±SD) of HIV-infected individuals was 54.7 ± 7.3 years and that of uninfected controls was 52.9 ± 8.8 years. The subgroups displayed characteristics representative of the main study (among HIV-infected individuals, 55% were male, 46% were men who have sex with men [MSM], and 55% were of Dutch origin; among HIV-uninfected controls, 67% were male, 67% were MSM, and 83% were of Dutch origin).
The study protocol was approved by local ethics review committee and registered at ClinicalTrials.gov (identifier NCT01466582; available at: http://www.clinicaltrials.gov). All participants provided written informed consent.
Flow Cytometry
PBMCs were isolated from fresh blood specimens, using Ficoll-Isopaque density gradient centrifugation, and these freshly isolated cells were used for immune phenotyping. The following directly conjugated monoclonal antibodies were used for cell surface marker staining (for 30 minutes at 4°C in the dark): CD45RA PE-Cy7, CD4 PE-Cy7, HLA-DR FITC, CD38 PE, CD25 APC, CD28 PerCP Cy5.5, CD27 PerCP Cy5.5, CD3 V500, CD57 APC, and CD8 Pacific Blue (BD Biosciences, Breda, the Netherlands); and CD31 APC, PD-1 PE, CD27 APCeFluor780, CD127 APCeFluor780, and CD4 APCeFluor780 (eBioscience, Vienna, Austria). Fluorescence was measured with the FACS Canto II (BD Biosciences, Breda, the Netherlands). The proportion of cells expressing each marker were determined using FlowJo V10 (FlowJo, Ashland, Oregon).
CMV-Specific T-Cell Responses
CMV-specific T-cell responses to overlapping peptide pools were assessed in cryopreserved PBMC samples, based on the protocol described by Lamoreaux et al [14]. After thawing, cells were left to rest overnight at 37°C and 5% CO2 in a humidified atmosphere. Subsequently, cells were stimulated with 15–amino acid overlapping peptides that span the entire CMV protein pp65 (0.15 μg/mL) and EI-1 (0.31 μg/mL) [15] in the presence of anti-CD107a-FITC (eBiosciences), brefeldin A and GolgiStop (BD Biosciences), and anti-CD28 and anti-CD29 as costimulation for 6 hours. As a positive control, cells were stimulated with PMA and ionomycin for 4 hours. Cells were then stained with anti-CD4-PECy5.5 (Life Technologies, Waltham, Massachusetts) and anti-CD3-V500 and anti-CD8-PB (BD Biosciences). For intracellular staining, cells were permeabilized with the BD Cytofix/Cytoperm kit and then stained with anti-interleukin 2 (IL-2)–PE, anti-tumor necrosis factor α (TNF-α)–AF700, and anti-macrophage inflammatory protein 1β (MIP-1β)–PECy7 (BD Biosciences), as well as anti-interferon γ (IFN-γ)–APC-AF750 (Life Technologies). Finally, cells were fixed with Cellfix (BD Biosciences) and analyzed with the BD LSR II/Fortessa Flow cytometer (BD Biosciences). The proportion of cytokine-expressing cells were determined using FlowJo V10 (FlowJo).
CMV Antibody Titers
CMV antibody titers, as well as high-avidity antibody titers, were measured by an ELISA-Viditest anti-CMV immunoglobulin G (IgG) and IgG avidity assay (Vidia, Praha, Czech Republic), according to the manufacturer's protocol. For quantification, a standard curve was prepared by serial dilution of plasma from a CMV-seropositive individual.
Soluble CD163 (sCD163) and Soluble CD14 (sCD14) Levels
sCD163 and sCD14 levels were determined in stored plasma samples (−80°C) by an enzyme-linked immunosorbent assay (Human CD14 DuoSet and Human CD163 Duoset, R&D Systems, Minneapolis, Minnesota) according to the manufacturers' instruction.
Telomere and Single-Joint T-Cell Receptor Excision Circle (sjTREC) Polymerase Chain Reaction (PCR) Analysis
DNA was isolated from PBMCs, using the NucleoSpin Blood kit (Macherey-Nagel, Dueren, Germany) according to the manufacturers' instructions. Telomere length was measured by qPCR, using Sensifast SYBR no-ROX Mix (Bioline, Luckenwalde, Germany) and 5 ng of DNA, input as previously described [16], using the Tel-g ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT and Tel-c TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA at a final concentration of 400 nm. A qPCR for the single copy albumin gene was used as a control for input normalization, using the primers Alb-u CGGCGGCGGGCGGCGCGGGCTGGGCGGAAATGCTGCACAGAATCCTTG and Alb-d GCCCGGCCCGCCGCGCCCGTCCCGCCGGAAAAGCATGGTCGCCTGTT. The relative telomere length was estimated using a standard curve prepared from PBMCs obtained from a healthy blood bank donor.
sjTREC content was determined by nested PCR analysis as previously described [17]. Primers DTF6 AGAAGGCTCTGTCTAGTGTG and DTR61 TCTGACATTTGCTCCGTG were used to amplify sjTREC content in 500 ng of PBMC DNA, using Platinum Taq (Invitrogen). In the secondary PCR, sjTREC content from 6 µL of the first reaction was amplified using Platinum Taq (Invitrogen), SYBR Green (Invitrogen), and the primers DTF7 AGGCTCTGTCTAGTGTGATAAC and DTR66 TGACATGGAGGGCTGAAC. A plasmid containing the first-round PCR product was used as standard curve [17] to calculate the sjTREC content.
All PCR analyses were performed on the LightCycler 480 system (Roche, Basel, Switzerland).
Statistical Analysis
Differences in and associations between immunological markers and subject characteristics between HIV-infected ART recipients and uninfected controls were determined using the Student t test, the Pearson χ2 test, and univariable and multiple variable linear regression. Analyses were performed in SPSS 20 and GraphPad Prism 5.
RESULTS
Low CD4+ T-Cell Counts in HIV-Infected Individuals Despite Long-term Receipt of ART
A total of 189 participants, 95 HIV-infected individuals and 94 uninfected controls, from the AGEhIV cohort study [5] were analyzed in this study. All participants were >45 years of age, and the majority were male (Table 1). The control group contained significantly more participants of Dutch origin and MSM (Table 1). HIV-infected individuals had received ART for a mean duration (±SD) of 10.7 ± 5.8 years and had had an undetectable plasma viral load (<200 copies/mL) for mean cumulative duration (±SD) of 7.1 ± 4.3 years (Table 1). HIV-infected individuals had lower CD4+ T-cell counts, higher CD8+ T-cell counts, and a lower ratio of CD4+ to CD8+ T cells, compared with controls (Figure 1A and Supplementary Data). Immune phenotyping of PBMCs revealed that the proportional distribution of naive (CD45RA+CD27+), memory (CD45RA−CD27+), and effector-memory (CD27−) T cells did not differ between HIV-infected ART recipients and controls, either in the CD4 or CD8 compartments (Supplementary Data and Supplementary Data). Since there was an overrepresentation of Dutch MSM in the control group, immune phenotype data in Dutch MSM alone are displayed in Supplementary Data.
Table 1.
Subject Characteristics
| Characteristic | HIV-Infected ART Recipients (n = 94) | Uninfected Controls (n = 95) |
|---|---|---|
| Age, y | 55.6±7.5 | 56.3±8.0 |
| Male sex | 75 (80) | 79 (83) |
| Dutch origin | 62 (66) | 85 (89)a |
| MSM | 61 (65) | 72 (76)b |
| Duration of HIV infection, y | 13.1±6.2 | NA |
| ART naive at ART initiation | 65 (69) | NA |
| Duration of ART, y | 10.7±5.8 | NA |
| Duration of undetectable viral load, y | 7.1±4.3 | NA |
| CD4+ T-cell count, cells/µL | ||
| Nadir | 165±111 | NA |
| At time of ART initiation | 291±194 | NA |
| Recovery after ART initiation | 460±267 | NA |
| CMV seropositive | 83 (89) | 81 (86) |
Data are mean value ± SD or no. (%) of subjects.
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; HIV, human immunodeficiency virus; MSM, men who have sex with men; NA, not applicable.
aP < .001, by the Pearson χ2 test.
bP < .05.
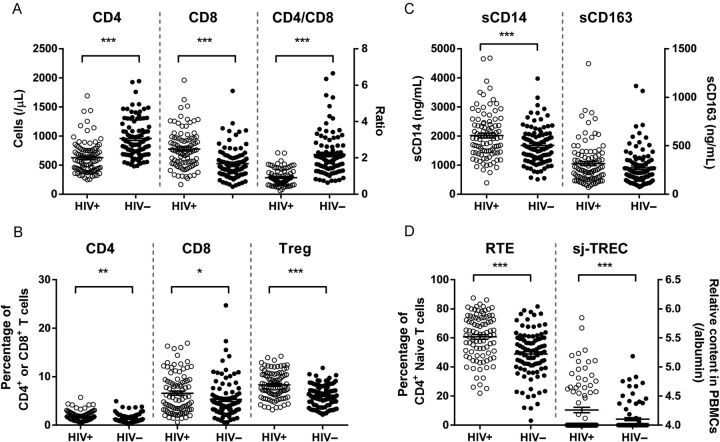
Figure 1.
T-cell phenotyping and immune activation in human immunodeficiency virus type 1 (HIV)–infected antiretroviral therapy (ART) recipients and uninfected controls. A, Absolute CD4+ T-cell counts, absolute CD8+ T-cell counts, and ratios of CD4+ to CD8+ T cells are shown for HIV-infected ART recipients and uninfected controls. B, T-cell activation was determined by the percentage of HLA-DR+CD38+ cells within the total CD4+ and CD8+ T-cell population, and the percentage of regulatory T cells (Tregs) was determined by the analysis of the proportion of CD4+CD25+CD127low cells among the CD4+ T cells. C, Monocyte activation and microbial translocation were determined by measuring soluble CD14 (sCD14) and soluble CD163 (sCD163) levels in plasma. D, Thymic output was determined by the proportion of CD31+ naive CD4+ T cells and single-joint T-cell receptor excision circle (sjTREC) content in peripheral blood mononuclear cells (PBMCs).
Absolute cell numbers of the subpopulations were significantly different between the HIV-infected individuals and uninfected controls, which can be explained by the low CD4+ T-cell count and high CD8+ T-cell count in the HIV-infected individuals (Figure 1A and Supplementary Data).
Higher Immune Activation Is Associated With Low CD4+ T-Cell Counts in HIV-Infected ART Recipients
We observed that the percentages of activated (CD38+HLA-DR+) cells in the CD4+ and the CD8+ T-cell population were higher in HIV-infected individuals (Figure 1B and Supplementary Data). A higher percentage of activated CD4+ T cells was strongly associated with a lower CD4+ T-cell count (Beta = −0.41, P = 5.7 × 10−5) and reduced CD4+ T-cell recovery after starting ART (Beta = −0.37, P = 3.1 × 10−4). We observed a significantly higher level of sCD14 (P = .001) and a trend toward higher levels of sCD163 (P = .13) in plasma specimens from HIV-infected individuals, compared with controls (Figure 1C and Supplementary Data). The sCD14 level was positively associated with the percentage of activated CD4+ T cells (Beta = 0.24, P = .023; Supplementary Data). Similarly, sCD163 levels were strongly associated with activation of CD4+ T cells (Beta = 0.51, P = 1.9 × 10−7) and CD8+ T cells (Beta = 0.32, P = .002; Supplementary Data).
The percentage of regulatory T cells (Tregs; CD4+CD25+CD127low) in HIV-infected ART recipients was higher, compared with that for negative controls (Figure 1B and Supplementary Data). The higher proportion of Tregs was positively associated with the percentage of CD4+CD38+HLA-DR+ cells (Beta = 0.41, P = 4.3 × 10−5) and plasma levels of sCD14 (Beta = 0.30, P = .004) but not with the percentage of CD8+CD38+HLA-DR+ T cells. In controls, the proportion of Tregs was negatively associated with age (Beta = −0.28, P = .007).
Thymic Output in HIV-Infected ART Recipients Is Not Associated With Immune Activation and CD4+ T-Cell Counts
Diminished thymic function might limit restoration of the number of circulating CD4+ T cells during ART. We observed a higher percentage of CD31+ cells within the CD4+ naive T-cell population and higher sjTREC content in PBMCs among individuals with HIV infection as compared to those without HIV infection (Figure 1D ). The sjTREC content in PBMCs was strongly associated with the percentage of CD31+ naive CD4+ cells (Beta = 0.38, P = .0003) and the percentage of CD4+ naive T cells (Beta = 0.35, P = .0009) in treated HIV-infected individuals, whereas in controls the sjTREC content in PBMCs was associated with the percentage of CD8+ naive T cells (Beta = 0.37, P = .0003; Supplementary Data). However, no associations between sjTREC content and CD4+ T-cell recovery during ART or immune activation were observed.
T-Cell Replicative History in HIV-Infected ART Recipients Is Associated With Immune Activation
Telomere shortening is a hallmark of cell proliferation [18]. During normal aging, shortening of telomeres is consistently observed in cells from the immune system, and it is more evident during chronic inflammation and untreated HIV infection [19]. We observed that PBMCs from HIV-infected ART recipients had shorter telomeres than those from controls (Figure 2A ). Notably, during ART, shorter telomeres were associated with higher levels of CD4+ activated T cells (Beta = −0.30, P = .004) and higher levels of the monocyte activation markers sCD14 and sCD163 (Beta =−0.27, P = .010; Supplementary Data). In controls, shorter telomeres were associated with higher levels of CD8+ activated T cells (Beta = −0.30, P = .004) and lower percentages of CD4+ memory T cells (Beta = 0.33, P = .002; Supplementary Data).
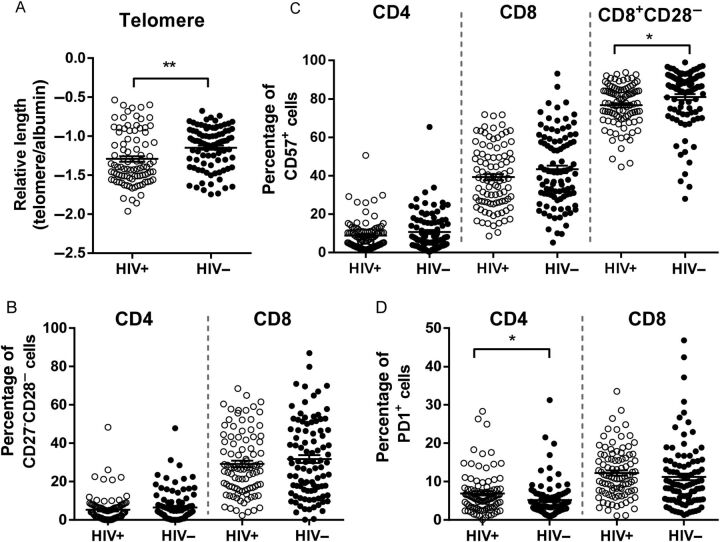
Figure 2.
Shorter telomeres but normalization of immune senescence in human immunodeficiency virus type 1 (HIV)–infected individuals receiving long-term antiretroviral therapy. A, Telomere length in peripheral blood mononuclear cells was analyzed as a measure of historical cell proliferation. B and C, The percentage of late-differentiated and immune-senescent T cells was determined by the lack of CD27 and CD28 expression on CD4+ and CD8+ T cells (B) and the expression of CD57 on CD4+ and CD8+ T cells (C). Additionally, the percentage of CD57+ cells in the CD8+CD28− cell population is given (C). D, The level of T-cell exhaustion is expressed as the percentage PD-1+ cells within the CD4+ and CD8+ T-cell population.
Normalization of CD4+ and CD8+ T-Cell Senescence in HIV-Infected ART Recipients
Healthy aging is characterized by the accumulation of terminally differentiated T cells, and this process can be accelerated by persistent immune activation, especially during HIV infection [9, 13, 20]. Here, we observed that the percentage of late differentiated (CD27−CD28−) and CD57-expressing CD4+ and CD8+ T cells was not different between HIV-infected individuals and controls (Figure 2B and 2 C and Supplementary Data).
Recently, it has been reported that the proportion of CD57-expressing cells among CD28−CD8+ T cells is decreased during HIV infection, whereas this population increases during healthy aging and CMV infection [21, 22]. We indeed observed that the proportion of CD57-expressing cells among CD28−CD8+ T cells was decreased in the HIV-infected population (Figure 2C and Supplementary Data), but no differences in CD57 expression among the CD28−CD4+ T cells was observed (Supplementary Data).
Furthermore, we observed that PD-1 expression was higher among HIV-infected individuals, compared with controls, among CD4+ T cells but not among CD8+ T cells (Figure 2D and Supplementary Data). The percentage of PD-1–positive cells among CD4+ T cells was associated with the percentage of Tregs in HIV-infected individuals (Beta = 0.29, P = .005). Activated Tregs express high levels of PD-1 [23], and therefore the increased PD-1 levels might be explained by the increase in Tregs in HIV-infected ART recipients.
Higher CMV Antibody Titers but Normal CMV-Specific T-Cell Responses in HIV-Infected ART Recipients
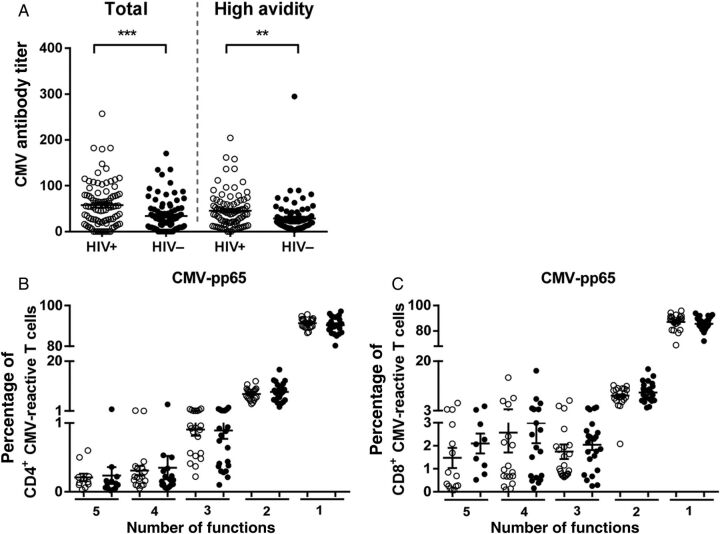
CMV infection and the CMV-specific T-cell response have been associated with immune senescence in aging individuals [24]. In our study population, 89% of HIV-infected individuals receiving ART and 86% of controls were positive for anti-CMV IgG antibodies. We observed higher anti-CMV total and high-avidity antibody titers in treated HIV-infected individuals (Figure 3A ). The percentage of CD4+ or CD8+ T cells that responded to CMV pp65 or IE-1 antigens was not different between HIV-infected individuals and controls (Supplementary Data A). Furthermore, no correlation between CMV-pp65– or CMV-IE-1–specific T cells and age was observed (data not shown). Polyfunctionality of CMV-specific cells did not differ between HIV-infected ART recipients and controls (Figure 3B and 3 C and Supplementary Data B). The ability of CD4+ and CD8+ T cells to produce IFN-γ, IL-2, TNF-α, or MIP-1β upon stimulation with CMV-pp65 or CMV-IE-1 peptides did not differ between individuals with treated HIV infection and controls (Supplementary Data).
Figure 3.
Higher cytomegalovirus (CMV) antibody titers but normal frequencies of functional CMV-reactive T cells in human immunodeficiency virus type 1 (HIV)–infected antiretroviral therapy (ART) recipients. A, Total anti-CMV immunoglobulin G (IgG) titers and high-avidity antibody titers were determined in CMV-positive HIV–infected ART recipients and uninfected controls. T-cell responses to CMV-specific peptides (pp65) were characterized by the analysis of cytokine production (interferon γ, tumor necrosis factor α, interleukin 2, and macrophage inflammatory protein 1β) and the degranulation marker CD107a in 22 HIV-infected ART recipients and 24 uninfected controls. Polyfunctionality of the CMV-specific cells was determined by the ability of the cells to produce different cytokines and the expression of the degranulation marker (CD107a). B and C, The proportion of cells that displayed ≥1 function of the total number of CMV-pp65 reactive CD4+ (B) and CD8+ (C) T cells is given. Open circles denote HIV-infected ART recipients, and closed circles denote uninfected controls.
Immune Senescence Independently Associates With T-Cell Activation Levels
Multivariate linear regression models to determine predictors of CD4+ or CD8+ T-cell senescence, as measured by the proportion of CD27−CD28− cells or the proportion of CD57+ cells, were used. Regression models included parameters selected by univariable linear regression analysis (age, CD4+ T-cell count, CD8+ T-cell count, ratio of CD4+ to CD8+ T cells, CMV high-avidity antibody titer, CMV antibody titer, activated CD4+ and CD8+ T cells, CD4+PD-1+ cells, and Treg percentage). Using a backward approach and optimization by hand, models were minimized to include only the most significant predictors. CD4+ T-cell activation (ie, percentage of CD38+HLA-DR+ cells) was strongly associated with the proportion of CD4+CD57+ T cells in HIV-infected individuals receiving ART, whereas CD8+ T-cell count was associated with CD4+ T-cell senescence (ie, percentage of CD27−CD28− cells or percentage of CD57+ cells) in controls (Table 2). Immune senescence in CD8+ T cells, as demonstrated by the proportion of CD57+ and CD27−CD28− cells, in HIV-infected ART recipients was dependent on multiple variables: age, CD4+ T-cell count, CD8+ T-cell count, and CD8+ T-cell activation (ie, percentage of CD38+HLA-DR+ cells). In controls, CD8+ T-cell senescence (ie, CD57+ and CD27−CD28− cells) was determined by CD8+ T-cell count (Table 2).
Table 2.
Multivariable Linear Regression Model for CD4+ and CD8+ T-Cell Senescence
| Variable | HIV-Infected ART Recipients |
Uninfected Controls |
||||
|---|---|---|---|---|---|---|
| B (95% CI) | Beta | P Value | B (95% CI) | Beta | P Value | |
| CD4+CD27-CD28- | ||||||
| Age | 0.14 (−.05–.34) | 0.15 | .15 | 0.11 (−.08–.30) | 0.11 | .24 |
| CD8+ T-cell count | 0.001 (−.003–.006) | 0.07 | .50 | 0.013 (.007–.019) | 0.42 | 1.7 × 10−5 |
| CD4+CD38+HLA-DR+ | 1.82 (.39–3.24) | 0.26 | .013 | 1.11 (.65–2.86) | 0.12 | .21 |
| CMV antibody titer | 0.012 (−.018–.042) | 0.081 | .43 | 0.052 (.007–.096) | 0.21 | .022 |
| CD4+CD57+ | ||||||
| Age | 0.112 (−.093–.32) | 0.11 | .28 | 0.065 (−0.17–.30) | 0.054 | .57 |
| CD8+ T-cell count | 0.001 (−.003–.006) | 0.060 | .53 | 0.013 (.006–.020) | 0.35 | .0006 |
| CD4+CD38+HLA-DR+ | 2.95 (1.43–4.47) | 0.39 | .0002 | 2.22 (.051–4.39) | 0.20 | .045 |
| CD8+CD27-CD28- | ||||||
| Age | 0.58 (.18–.98) | 0.27 | .005 | 0.53 (.078–.99) | 0.21 | .022 |
| CD4+ T-cell count | −0.015 (−.027 to −.003) | −0.24 | .015 | −0.004 (−.016–.008) | −0.064 | .51 |
| CD8+ T-cell count | 0.014 (.004–.024) | 0.28 | .005 | 0.03 (.02–.05) | 0.44 | 2.8 × 10−5 |
| CMV antibody titer | 0.06 (−.005–.12) | 0.17 | .07 | 0.12 (.01–.23) | 0.2 | .03 |
| CD8+CD38+HLA-DR+ | 1.04 (.27–1.81) | 0.25 | .009 | 0.34 (−.63–1.31) | 0.07 | .48 |
| CD8+CD57+ | ||||||
| Age | 0.43 (.045–.82) | 0.2 | .03 | 0.33 (−.13–.79) | 0.138 | .16 |
| CD4+ T-cell count | −0.020 (−.032 to −.008) | −0.33 | .001 | −0.003 (−.015–.009) | −0.050 | .63 |
| CD8+ T-cell count | 0.014 (.005–.023) | 0.29 | .004 | 0.026 (.011–.041) | 0.36 | .001 |
| CD8+CD38+HLA-DR+ | 1.46 (.73–2.20) | 0.37 | .0002 | 0.56 (−.42–1.54) | 0.12 | .26 |
Multivariate linear regression models included parameters selected by univariable linear regression analysis (age, CD4+ T-cell count, CD8+ T-cell count, ratio of CD4+ to CD8+ T cells, CMV high-avidity antibody titer, CMV antibody titer, activated CD4+ and CD8+ T cells, CD4+PD-1+ cells, and Treg percentage). Using a backward approach and optimization by hand, models were minimized to include only the most significant predictors.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; HIV, human immunodeficiency virus; Treg, regulatory T cell.
DISCUSSION
HIV-infected individuals with suppressed viremia during ART have higher rates of age-associated diseases and, on average, a shorter life expectancy, compared with uninfected persons of the same age [4, 5, 25]. Here, we analyzed several immunological factors that could plausibly contribute to excess comorbidity in HIV disease in the AGEhIV Cohort Study, which was prospectively designed to recruit HIV-infected and HIV-uninfected adults who had comparable socioeconomic and health behavior backgrounds.
As expected our HIV-infected individuals had lower CD4+ T-cell counts, higher CD8+ T-cell counts, and lower ratios of CD4+ to CD8+ T cells than controls, similar to previous reports that normal CD4+ T-cell levels are not achieved in many effectively treated HIV-infected individuals [26, 27]. Upon initiation of ART, the increase in CD4+ T-cell count is initially accomplished by redistribution and peripheral expansion of memory cells [28, 29], followed by thymic production of naive CD4+ T cells [30, 31]. Poor CD4+ T-cell recovery, especially in older HIV-infected individuals receiving ART, might be a reflection of impaired thymic function [32]. Here, we observed higher levels of sjTREC and higher percentages of CD31+ naive CD4+ cells in HIV-infected individuals as compared to controls, indicative of a functional thymus and confirming previous observations [33]. The sjTREC content and the proportion of CD31+ naive CD4+ T cells were not associated with CD4+ T-cell counts, suggesting that poor CD4+ T-cell recovery was not the result of diminished thymic function.
Although ART drastically reduces immune activation, HIV-infected individuals still show signs of persistent immune activation and inflammation during ART [34, 35]. Increased levels of immune activation, as reflected by higher sCD14 levels in plasma and higher percentages of CD38+HLA-DR+ CD4+ and CD8+ T cells, were observed in our HIV-infected ART recipients, compared with controls. In HIV-infected individuals, CD4+ T-cell activation was inversely associated with CD4+ T-cell count and CD4+ T-cell recovery. Interestingly, increased immune activation (ie, percentage of CD4+CD38+HLA-DR+ and plasma levels of sCD14 and sCD163) was also associated with shorter telomeres in HIV-infected ART recipients. In agreement with previous studies [36–38], our data suggest that chronic inflammation associated with HIV disease (adaptive and innate immune system) drives excess activation and proliferation of T cells, which in turn leads to telomere shortening and ultimately to poor immune recovery and the immune-senescent phenotype.
Aging and CMV infection have been associated with immune-senescent (ie, CD57 expression or a lack of CD27/CD28 expression) T cells [13, 24, 39]. In contrast to a previous study [9], no differences in immune-senescent CD4+ and CD8+ T cells were observed between treated HIV-infected individuals and controls. However, the proportion of CD57-expressing cells within the CD8+CD28− population was significantly decreased in HIV-infected ART recipients, indicating that HIV infection, in contrast to CMV infection and aging, inhibits terminal differentiation within CD28−CD8+ T cells, as reflected by a decrease of CD57 expression [21, 22].
Although CMV IgG titers were higher in HIV-infected individuals [40, 41], no differences in the proportion and polyfunctionality of CMV-specific T cells between HIV-infected individuals and controls were observed. This contradicts findings from previous studies [9, 10]. Differences in age, duration of HIV infection, and time of effective ART may influence the size of the CMV-specific T-cell response and explain differences in study outcomes.
Regulatory T cells play an important role in the maintenance of immune homeostasis by limiting immune activation and suppressing effector functions of immune cells, thus protecting host tissues from immune-mediated damage [42, 43]. Here, HIV-infected individuals receiving ART had a higher Treg percentage, which was associated with increased immune activation, confirming previous observations [44–46]. This suggests that the Treg percentage increased as a consequence of increased immune activation.
PD-1 expression on CD4+ T cells was also increased in HIV-infected individuals, compared with controls, and was associated with T-cell activation. It has been reported that the inflammatory environment of HIV disease drives excess Treg function and upregulation of a number of counterregulatory pathways, including excess production of IL-10 and upregulation of PD-1 on T cells [47, 48]. A remarkably similar set of observations has been reported in cancer tissues and may explain the significant success that blocking these pathways has had on T-cell function and disease outcomes in that setting [49].
In conclusion, CD4+ T-cell counts in HIV-infected individuals remain lower than in uninfected controls, despite long-term ART. Immune activation levels were likewise increased in HIV-infected ART recipients, which was also reflected in an increased Treg percentage. T-cell activation in the HIV-infected group is most likely driven by monocyte activation and bacterial translocation, as demonstrated by sCD14 and sCD163. Furthermore, no differences in T-cell senescence (ie, proportion of CD27−CD28− or CD57+ cells) and CMV-responsive T cells were observed between HIV-infected ART recipients and uninfected controls. However, a strong independent association between immune activation and immune senescence is still observed in HIV-infected individuals despite long-term use of ART.
STUDY GROUP MEMBERS
Scientific Oversight and Coordination
Academic Medical Center (AMC), Department of Global Health/Amsterdam Institute for Global Health and Development (AIGHD): P. Reiss (principal investigator), F. W. N. M. Wit, M. van der Valk, J. Schouten, K. W. Kooij, R. A. Van Zoest, and B. C. Elsenga; and Public Health Service Amsterdam, Infectious Diseases Research Cluster: M. Prins (co–principal investigator), M. Martens, S. Moll, J. Berkel, M. Totté, G. R. Visser, and S. Kovalev.
Data Management
HIV Monitoring Foundation: S. Zaheri, M. M. J. Hillebregt, Y. M. C. Ruijs, D. P. Benschop, and P. Reiss.
Project Management and Administrative Support
AIGHD: F. R. Janssen, M. Heidenrijk, W. Zikkenheiner, and L. Boumans.
Central Laboratory Support
AMC, Laboratory for Viral Immune Pathogenesis and Department of Experimental Immunology: N. A. Kootstra, A. M. Harskamp-Holwerda, I. Maurer, M. M. Mangas Ruiz, A. F. Girigorie, and B. Boeser-Nunnink.
Participating HIV Physicians and Nurses
AMC, Division of Infectious Diseases: S. E. Geerlings, M. H. Godfried, A. Goorhuis, J. W. R. Hovius, F. J. B. Nellen, J. T. M. van der Meer, T. van der Poll, J. M. Prins, P. Reiss, M. van der Valk, W. J. Wiersinga, F. W. N. M. Wit; J. van Eden, A. M. H. van Hes, M. Mutschelknauss, H. E. Nobel, F. J. J. Pijnappel, and A. M. Westerman.
Other Collaborators
AMC, Department of Cardiology: J. de Jong and P. G. Postema; AMC, Division of Endocrinology and Metabolism: P. H. L. T. Bisschop and M. J. M. Serlie; Free University Medical Center Amsterdam, Division of Endocrinology and Metabolism: P. Lips; AMC, Department of Gastroenterology: E. Dekker; AMC, Division of Geriatric Medicine: S. E. J. A. de Rooij; AMC, Division of Nephrology: J. M. R. Willemsen and L. Vogt; AMC, Department of Neurology: J. Schouten, P. Portegies, B. A. Schmand, G. J. Geurtsen, J. A. ter Stege, and M. Klein Twennaar; AMC, Department of Nuclear Medicine: B. L. F. van Eck-Smit and M. de Jong; AMC, Division of Clinical Oncology: D. J. Richel (retired); AMC, Department of Ophthalmology: F. D. Verbraak and N. Demirkaya; AMC, Department of Psychiatry: I. Visser and H. G. Ruhé; AMC, Department of Medical Psychology: P. T. Nieuwkerk; AMC, Department of Pulmonary Medicine: R. P. van Steenwijk and E. Dijkers; AMC, Department of Radiology: C. B. L. M. Majoie, M. W. A. Caan, and T. Su; AMC, Department of Gynecology: H. W. van Lunsen and M. A. F. Nievaard; AMC, Division of Vascular Medicine: B. J. H. van den Born and E. S. G. Stroes; and HIV Vereniging Nederland: W. M. C. Mulder.
Supplementary Material
Notes
Acknowledgments. We thank all study participants, without whom this research would not be possible.
V. C. J. and M. J. were responsible for experimental design, conduct of experiments, data analysis, data interpretation, and manuscript writing; F. W. N. M. W., J. S., T. B., S. G. D., and P. R. were responsible for study design, data analysis, data interpretation, and manuscript writing; I. M. and A. M. H. were responsible for experimental design and conduct of experiments; M. P. and E. M. M. v. L. were responsible for study design and manuscript writing; N. A. K. was responsible for study design, experimental design, data analysis, data interpretation, and manuscript writing.
Financial support. This work was supported by the AIDS Fonds (grant 2010039). The AGEhIV Cohort Study was supported by The Netherlands Organisation for Health Research and Development (ZonMW) (grant 300020007), AIDS Fonds (grant 2009063), Gilead Sciences, ViiV Healthcare, Janssen Pharmaceutica, Bristol-Myers Squibb, and Merck.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
AGEhIV Study Group:
Viviana Cobos Jiménez, Ferdinand W. N. M. Wit, Maaike Joerink, Irma Maurer, Agnes M. Harskamp, Judith Schouten, Maria Prins, Ester M. M. van Leeuwen, Thijs Booiman, Steven G. Deeks, Peter Reiss, and Neeltje A. Kootstra
References
- 1.van Sighem AI, van de Wiel MA, Ghani ACet al. Mortality and progression to AIDS after starting highly active antiretroviral therapy. AIDS 2003; 17:2227–36. [DOI] [PubMed] [Google Scholar]
- 2.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol 2013; 177:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulet JL, Fultz SL, Rimland Det al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007; 45:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guaraldi G, Orlando G, Zona Set al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 5.Schouten J, Wit FW, Stolte IGet al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev 2011; 10:319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appay V, Fastenackels S, Katlama Cet al. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011; 25:1813–22. [DOI] [PubMed] [Google Scholar]
- 10.Naeger DM, Martin JN, Sinclair Eet al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dock JN, Effros RB. Role of CD8T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis 2011; 2:382–97. [PMC free article] [PubMed] [Google Scholar]
- 12.Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep 2010; 7:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenchley JM, Karandikar NJ, Betts MRet al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003; 101:2711–20. [DOI] [PubMed] [Google Scholar]
- 14.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc 2006; 1:1507–16. [DOI] [PubMed] [Google Scholar]
- 15.Kern F, Faulhaber N, Frommel Cet al. Analysis of CD8T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol 2000; 30:1676–82. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrando-Martinez S, Franco JM, Ruiz-Mateos Eet al. A reliable and simplified sj/beta-TREC ratio quantification method for human thymic output measurement. J Immunol Methods 2010; 352:111–7. [DOI] [PubMed] [Google Scholar]
- 18.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345:458–60. [DOI] [PubMed] [Google Scholar]
- 19.Hodes RJ. The effects of aging on lymphocyte development and function: introduction. Springer Semin Immunopathol 2002; 24:1–5. [DOI] [PubMed] [Google Scholar]
- 20.Papagno L, Spina CA, Marchant Aet al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol 2004; 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SA, Sinclair E, Hatano Het al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 2014; 9:e89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SA, Sinclair E, Jain Vet al. Low proportions of CD28- CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis 2014; 210:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol 2006; 176:2808–16. [DOI] [PubMed] [Google Scholar]
- 24.Koch S, Larbi A, Ozcelik Det al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci 2007; 1114:23–35. [DOI] [PubMed] [Google Scholar]
- 25.Hasse B, Ledergerber B, Furrer Het al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann GR, Perrin L, Pantaleo Get al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med 2003; 163:2187–95. [DOI] [PubMed] [Google Scholar]
- 27.Serrano-Villar S, Sainz T, Lee SAet al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucy RP, Hockett RD, Derdeyn CAet al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest 1999; 103:1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker RE, Carter CS, Muul Let al. Peripheral expansion of pre-existing mature T cells is an important means of CD4+ T-cell regeneration HIV-infected adults. Nat Med 1998; 4:852–6. [DOI] [PubMed] [Google Scholar]
- 30.Douek DC, McFarland RD, Keiser PHet al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998; 396:690–5. [DOI] [PubMed] [Google Scholar]
- 31.Franco JM, Rubio A, Martinez-Moya Met al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood 2002; 99:3702–6. [DOI] [PubMed] [Google Scholar]
- 32.Teixeira L, Valdez H, McCune JMet al. Poor CD4T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS 2001; 15:1749–56. [DOI] [PubMed] [Google Scholar]
- 33.McCune JM, Loftus R, Schmidt DKet al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest 1998; 101:2301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cockerham LR, Siliciano JD, Sinclair Eet al. CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One 2014; 9:e110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chevalier MF, Petitjean G, Dunyach-Remy Cet al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog 2013; 9:e1003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donovan A, Pantell MS, Puterman Eet al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One 2011; 6:e19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasa S, Fitch KV, Petrow Eet al. Soluble CD163 is associated with shortened telomere length in HIV-infected patients. J Acquir Immune Defic Syndr 2014; 67:414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong JY, De Vivo I, Lin X, Fang SC, Christiani DC. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One 2014; 9:e87348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasto S, Colonna-Romano G, Larbi A, Wikby A, Caruso C, Pawelec G. Role of persistent CMV infection in configuring T cell immunity in the elderly. Immun Ageing 2007; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parrinello CM, Sinclair E, Landay ALet al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 2012; 205:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunt SJ, Lee S, D'Orsogna L, Bundell C, Burrows S, Price P. The use of humoral responses as a marker of CMV burden in HIV patients on ART requires consideration of T-cell recovery and persistent B-cell activation. Dis Markers 2014; 2014:947432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol 2007; 7:875–88. [DOI] [PubMed] [Google Scholar]
- 43.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30:636–45. [DOI] [PubMed] [Google Scholar]
- 44.Lim A, Tan D, Price Pet al. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. AIDS 2007; 21:1525–34. [DOI] [PubMed] [Google Scholar]
- 45.Piconi S, Trabattoni D, Gori Aet al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS 2010; 24:1991–2000. [DOI] [PubMed] [Google Scholar]
- 46.Weiss L, Piketty C, Assoumou Let al. Relationship between regulatory T cells and immune activation in human immunodeficiency virus-infected patients interrupting antiretroviral therapy. PLoS One 2010; 5:e11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon DS, Angin M, Hongo Tet al. CD4+ CD25+ regulatory T cells impair HIV-1-specific CD4T cell responses by upregulating interleukin-10 production in monocytes. J Virol 2012; 86:6586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Said EA, Dupuy FP, Trautmann Let al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med 2010; 16:452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.