Abstract
Background and study aims Colon capsule endoscopy (CCE) has been proposed as an alternative to colonoscopy for screening patients at average risk of colorectal cancer (CRC). A prospective national cohort was developed to assess relevance of CCE in real-life practice and its short- and long-term impacts on clinical management.
Patients and methods All patients who underwent a CCE in France were prospectively enrolled from January 2011 to May 2016 and reached annually by phone until May 2017. All CCE and colonoscopy reports were systematically collected.
Results During the study period, 689 CCEs were analyzed from 14 medical centers. Median follow-up time was 35 months [IQR: 12–50]. Indication for CCE was mainly for elderly patients (median age: 70 years, IQR: [61–79]) due to anesthetic or colonoscopy contraindication (n = 307; 44.6 %). Only 337 CCEs (48.9 %) were both complete and with adequate bowel preparation. Advanced neoplasia (adenoma with high-grade dysplasia or CRC) was diagnosed following 32 CCEs (4.6 %). Among patients who underwent colonoscopy or therapeutic surgery following CCE, 18.8 % of all advanced neoplasias (6/32) had not been diagnosed by CCE mainly due to technical issues. Performing a colonoscopy in the case of significant polyps or insufficient bowel cleansing or after an incomplete CCE allowed the diagnosis of 96.9 % of all identified advanced neoplasias (31/32).
Conclusions Outside the scope of academic trials, improvement is needed to increase the reliability of CCE as less than half were considered optimal i. e. complete with adequate bowel cleansing. Most of missed colonic advanced neoplasia were due to incomplete CCE with distal neoplasia location.
Introduction
Early detection of neoplastic and pre-neoplastic lesions of the colon mucosa is a key element to prevent mortality by colorectal cancer (CCR) 1 2 . Today, colonoscopy is the gold standard to explore colon mucosa 3 . Because it is invasive and presents procedure risks, it faces several issues regarding patient acceptability, contraindication to sedation, or technical limitation for whole-colon exploration 4 .
Colon capsule endoscopy (CCE) is a noninvasive technology based on the ingestion of a wireless capsule that allows acquisition of high-definition images of the colon mucosa 5 . It has been proposed as an alternative to colonoscopy for screening of average-risk colorectal cancer patients who show contraindications or are unwilling to undergo colonoscopy, and/or in cases of incomplete colonoscopy (cases of stenosis or insufficient bowel cleansing excluded) 6 7 8 . It has been demonstrated as a sure and effective tool to detect polyps at high risk of malignant development 9 10 . Diagnosis performance of second-generation CCE for detection of polyps ≥ 6 mm has been evaluated in several studies, with a sensitivity ranging from 79 % to 89 % and a specificity ranging from 64 % to 97 % 11 12 13 14 15 16 . However, clinical relevance of CCE in real-life practice and its short- and long-term impacts on clinical decisions have never been described. Indeed, CCE is of particular interest when colonoscopy cannot be performed, a clinical situation that could not be explored by clinical trials comparing CCE to colonoscopy. The aim of this study was thus to describe feasability, patients profile, results and the decision process that follows the use of CCE when performed in real-life.
To assess these questions, the results of the French National Observatory of Colon Capsule Endoscopy (ONECC), a systematic national observational cohort of patients who underwent second-generation CCE in France with a 5-year follow-up, are presented herein.
Patients and methods
Patient inclusion
During the study period, the use of CCE in France was only possible within the ONECC cohort piloted by the French Society of Digestive Endoscopy. Thus, all patients who underwent a CCE in France were enrolled in a prospective manner, from 2011 to 2016.
Ethical considerations
Written, informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (updated in 2013). This study was authorized by the National Commission for Data Protection and Liberties under the no. 1519762 and is registered on ClinicalTrials.gov (NCT 03533894) in accordance with the legislation in place at the time of the study.
Procedure
All patients ingested second-generation CCE (Pillcam Colon 2, Medtronic, Minnesota, United States) after a 1-day clear liquid diet and bowel preparation consisting of 4-L or 2-L (Moviprep split doses of polyethylene glycol based preparation ± bisacodyl 5 mg (given and as a rescue if CCE was not excreted). 40 mg sennosides was also given 2 days before CCE ingestion. After ingestion, the patient received a booster regimen of sodium-phosphate solution (45 mL and 30 mL) or, if contraindicated, polyethylene glycol (500 mL). CCE videos were then analyzed by a trained gastroenterologist using dedicated software (Rapid Reader 7.0, Medtronic, Minnesota, United States).
Data collection
The gastroenterologist that prescribed CCE implemented an online electronic Case-Report-Form (e-CRF) mentioning: demographic data, further indication of colon exploration, indication of CCE, polyp presence, location, and size, bowel cleansing grade, complication during recording, and completeness of colon exploration (defined by a CCE where all colon segments were declared to be seen). Were considered “significant”, polyps ≥ 6 mm in size and/or the association of ≥ 3 polyps 11 . Bowel cleanliness was graded according to the validated Leighton-Rex scale from 1 to 4 (1: Poor; 2: Fair; 3: Good; 4: Excellent) 17 . The gastroenterologist who analyzed the CCE also mentioned if he retained the indication to perform a colonoscopy following the CCE. There was one CCE reader per center, all with > 300 capsule endoscopy readings at the time of study (only small bowel capsule, as this was the first time CCE was used in France). All CCE readers followed a 2-day specific training for CCE reading. If a colonoscopy was performed, results were also reported. All CCE and colonoscopy reports were systematically collected and reviewed, and data analysis was performed only on complete data for which all reports were available to ensure data robustness. Diagnosis of neoplasia were all histologically confirmed.
Follow-up data
All enrolled patients were annually reached by phone during the study period and until May 2017. In cases of loss to follow-up, local administrative registers were systematically consulted to check for patient death at the end of follow-up.
Statistical analysis
Odds ratios were calculated and Fisher’s exact test performed using GraphPad Prism version 6.00 for Mac OS X (GraphPad Software, La Jolla, California, United States, www.graphpad.com ).
Results
Between 2011 and 2016, a total of 1,282 CCEs were performed in France. Complete data were available for 689 CCEs (53.7 %) ( Fig. 1 ) from 14 different medical centers (7 teaching hospitals, 7 general hospitals). The median (interquartile range; IQR) number of CCEs per center was 30 [22–45]. Median follow-up was 35 months (12–50). Follow-up was not possible for 107 patients (15.5 %). Median (IQR) age for patients undergoing a CEE was 70 years (61–79) years and the population concerned showed important comorbidities. The main indication for CCE was contraindication to anesthesia or colonoscopy (n = 307; 44.6 %). At the end of the study, 115 patients (16.7 %) were dead ( Table 1 ). Cause of death was reported in 26.1 % of cases (30/115), among which none were related to a colorectal neoplasia.
Fig. 1 .
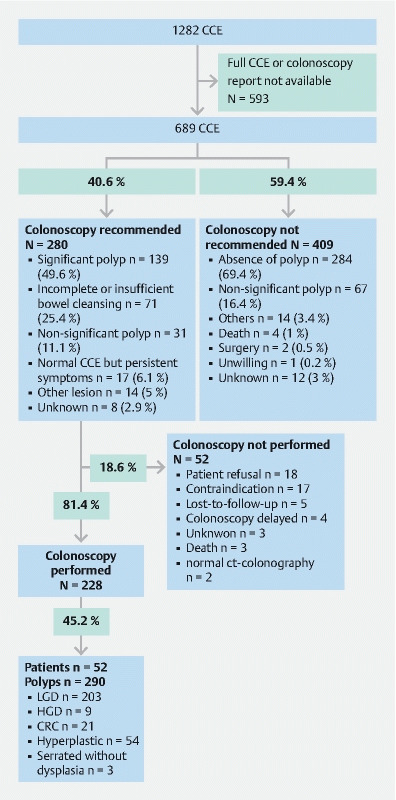
Study flowchart. Colonoscopy recommendation or absence of recommendation are detailed. CCE, colon capsule endoscopy; LGD, low-grade dysplasia; HGD, high-grade dysplasia; CRC, colorectal cancer.
Table 1. Patient characteristics.
| Patient characteristics | Values |
| Age, years | 70 [61–79] 1 |
| Sex ratio | 0.50 |
| Main indication for colon exploration, n (%) | |
|
148 (21.5) |
|
103 (15.0) |
|
155 (22.5) |
|
208 (30.2) |
|
23 (3.3) |
|
52 (7.5) |
| Main indication for colon capsule endoscopy | |
|
307 (44.6) |
|
217 (31.5) |
|
144 (20.9) |
|
21 (3) |
| Death at the end of follow-up | 115 (16.7) |
| Duration of follow-up (months) | 35 [12–50] 1 |
Median [interquartile range, IQR].
Bowel cleansing was considered as adequate (i. e. excellent or good) for 69.2 % (n = 477/689) of CCEs performed. A total of 442 (64.2 %) CCEs were considered complete among which 337 (48.9 %) were both complete and presenting adequate bowel preparation ( Table 2 ). Among the main polyethylene glycol-based preparation used, Moviprep preparation (Norgine, Amsterdam, Nederland) was not significantly associated with better adequate bowel cleansing than Colopeg (Recordati, Milan, Italy), (OR: 1.529; 95 %CI [0.9713– 2.406]). Polyps were identified in 298 CCE (43.2 %) and 187 CCE (27.1 %) allowed the detection of at least one significant polyp ( Table 3 ). Of note, among the CCEs that identified a significant polyp, 44.4 % (83/187) were described as incomplete or with insufficient bowel cleansing. In 18.3 % of cases, a non-polypoid lesion was described, concerning mainly diverticular disease (n = 92; 13.3 %; Table 3 ). No major complication related to CCE has been reported.
Table 2. Technical characteristics of second-generation colon capsule endoscopy (CCE) performed.
| Technical characteristics | Values, n (%) |
| Complete CCE (all colonic segments are seen) | 442 (64.2) |
| Complete CCE with excellent or good bowel cleansing | 337 (48.9) |
| Type of bowel cleansing regimen | |
|
484 (70.3) |
|
122 (17.7) |
|
41 (5.9) |
|
42 (6.1) |
| Bowel cleanliness | |
|
477 (69.2) |
|
190 (27.6) |
|
22 (3.2) |
CCE, colon capsule endoscopy.
Table 3. Main results of colon capsule endoscopy (CCE).
| Items | Values, n (%) |
| CCE with polyps | 298 (43.2) |
|
187 (27.1) |
|
111 (16.1) |
| Other lesion | 126 (18.3) |
|
92 (13.3) |
|
11 (1.6) |
|
4 (0.6) |
|
19 (2.8) |
CCE, colon capsule endoscopy.
In the majority of cases (409/689; 59.4 %), the gastroenterologist who completed the e-CRF did not recommend a colonoscopy following CCE, mainly due to the absence of polyps or the recording of a non-significant polyp (351/409; 85.8 %; Fig. 1 ). In this population for whom a colonoscopy was not recommended, 30.3 % (124/409) had an incomplete CCE. For those patients, the median age and indication for CCE were comparable to the whole cohort. Among patients who did not undergo a colonoscopy after the initial CCE, only one patient was reported with a CRC: one intramucosal cancer detected at colonoscopy 4 years after the initial CCE (colonoscopy performed after a sigmoid diverticulitis; Fig. 2 ).
Fig. 2 .
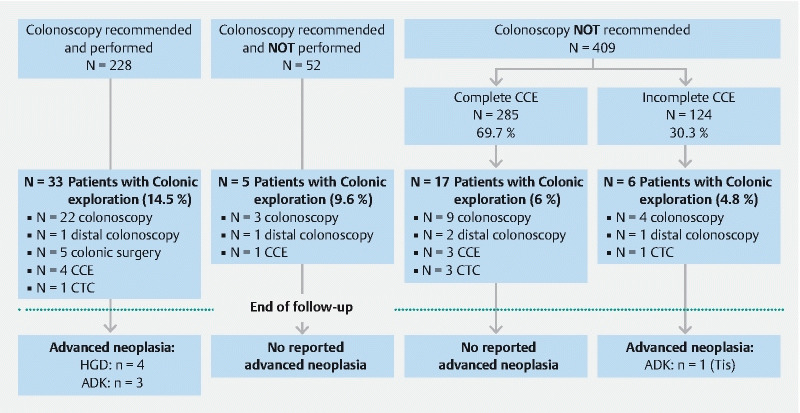
Summary of endoscopic exploration and diagnosis of advanced neoplasia during follow-up, according to initial management. CCE, colon capsule endoscopy; CTC, CT colonography; HGD, high-grade dysplasia; ADK, adenocarcinoma; Tis, intramucosal adenocarcinoma.
In 40.6 % of patients (280/689) a colonoscopy was recommended. Indications for colonoscopy are described in Fig. 1 . Among those with a recommendation to perform colonoscopy, 18.6 % (52/280) finally did not perform the examination mainly because of patient refusal (18/52; 34.6 %) or a confirmed medical contraindication to colonoscopy (17/52; 32.7 %). In 11.1 % of cases (31/280), the colonoscopy was recommended due to the diagnosis of a polyp on CCE even if the polyp did not meet criteria for significance. Overall, 27.9 % of CCEs (31/111) with a non-significant polyp gave rise to the indication for a colonoscopy.
When a colonoscopy was performed (n = 228) a polyp was diagnosed in 45.2 % of cases (103/228) representing 290 polyps among which 10.3 % (30/290) were advanced neoplasia (9 adenomas with high-grade dysplasia; 21 CRC). Interestingly, two CCEs led directly to surgery after the diagnosis of tumor without extra-colonic metastasis on thoracic and abdominal computed tomography scan. Surgery confirmed localized cancer in both. Among all 689 cases, 4.6 % of CCEs (32/689) were thus followed by the diagnosis of an advanced neoplasia (high grade or CRC), confirmed by surgery or colonoscopy. For these cases, the CCE and colonoscopy or surgery were concordant in 81.3 % of cases (26/32). The six cases for whom CCE results were non-significant and colonoscopy found advanced neoplasia are described in Table 4 . Importantly in four of six (66.7 %) of these misdiagnosed cases, capsule examination was incomplete and the advanced neoplasia was described as distal (sigmoid or rectum). In one case, the CCE and colonoscopy were concordant in the identification of a 5-mm polyp of the sigmoid colon, (i. e. a non-significant polyp according to the definition) that still justified a colonoscopy for the referent gastroenterologist with histology revealing an intramucosal CRC. In the last case, a lesion characterized as a voluminous lipoma of about 3 cm was described in the colonic region where a voluminous CRC was diagnosed at colonoscopy, raising the question of lesion misdiagnosis on CCE.
Table 4. Description of patients with advanced neoplasia at colonoscopy not detected at colon capsule endoscopy (CCE).
| Patient | Age (years) | CCE result | Bowel cleansing | Indication to complete CCE by colonoscopy | Advanced neoplasia location | Histology |
| 1 | 80 | No polyp | Excellent | CCE incomplete (rectum) | Rectum | Intramucosal adenocarcinoma |
| 2 | 65 | 5-mm polyp Right colon | Fair | Insufficient preparation | Rectum | Invasive adenocarcinoma |
| 3 | 50 | No polyp | Poor | Insufficient preparation | Rectum | Invasive adenocarcinoma |
| 4 | 68 | No polyp | Poor | Insufficient preparation | Sigmoid | Invasive adenocarcinoma |
| 5 | 84 | 5-mm polyp Sigmoid | Poor | CCE incomplete (rectum) | Sigmoid | Invasive adenocarcinoma |
| 6 | 74 | 23-mm lipoma Right colon |
Good | Unspecified | Right colon | Invasive adenocarcinoma |
CCE, colon capsule endoscopy.
Overall colonoscopy and CCE were concordant (polyp size and location) in 48.2 % of cases (110/228). For patients with a non-significant polyp at CCE and who underwent colonoscopy (n = 44), only one polyp (1/44; 2.3 %) corresponded to an advanced neoplasia (rectal CRC) after a CCE with insufficient bowel cleansing. Performing a colonoscopy after CCE in the case of significant polyps or insufficient bowel cleansing or after an incomplete CCE allowed the diagnosis of 96.9 % of all identified advanced neoplasias (31/32).
Discussion
In the ONECC cohort, CCE was mainly used for elderly and fragile patients with contraindication to colonoscopy, which may represent one main indication for colon capsule in order to avoid sedation or anesthesia in these patients. About half of CCEs identified a polyp and a colonoscopy was recommended for 40.6 % of all CCEs performed. About 5 % of CCEs led to a diagnosis of advanced neoplasia with a concordance between capsule/invasive colonic explorations of 81.3 %. However, less than half of all CCEs were considered optimal, i. e. complete with adequate bowel cleansing. False-negative CCE cases were mainly related to incomplete CCEs with distal CRC.
The aim of this study was not to assess the diagnosis performance of CCE given the fact that all patients did not perform the gold standard diagnostic test (colonoscopy); however, this is the first population-based, real-life study of CCE with long-term prospective follow-up. With patient enrollment coming from teaching hospitals and general hospitals, this study gives a good overview of how CCE can be used in clinical practice, and how it can impact patient management outside the scope of academic comparative controlled trials.
As confirmed by the present results, the main clinical situation of interest for CCE use is when colonoscopy cannot be performed (incomplete or contraindicated), a clinical situation that cannot be evaluated in a previous study when CCE was compared to colonoscopy. In such situations, CCE has already demonstrated superiority against CT colonography, the other alternative for noninvasive colonic exploration 15 18 19 . The ONECC cohort further showed reassuring results for CCE use in this population with high concordance between CCE and invasive colon exploration for high-grade dysplasia or CRC.
Moreover, in this real-life cohort, use of CCE showed specific interests in terms of management, demonstrating the possibility to perform colonic surgery directly after obvious tumor identification on CCE, with an increasing patient care efficiency. Of note, in about 10 % of cases, colonoscopy was recommended by practitioners despite the presence of non-significant polyps during a reassuring complete CCE with adequate bowel cleansing. This suggests that polyp size and number may not be the only way to assess the relevance of performing a colonoscopy after CCE. Clinical parameters, patient and gastroenterologist risk perception, and the optical aspect of the polyp on CCE, particularly a suspicious aspect, contribute to the decision-making process. Thus, it might be of interest to systematically assess the degree of suspicion of malignancy on CCE reports based on the polyp images obtained to help clinical decision in cases where size and number may not be sufficient. More precisely, in this cohort, this could have helped avoid the one missed case of high-grade dysplasia from the 5-mm isolated polyp identified on CCE. Developing a potential malignancy qualitative scale may be of interest to describe polyps seen on CCE in order to homogenize descriptions.
A limitation of the present study relates to missing data, as about 15 % of patients were lost to follow-up and complete CCE and colonoscopy reports were not available for half of the CCEs performed in France, and thus, not included in the analysis. Death causes were also not all known and some deaths related to colonic neoplasia or new diagnosis of CRC may have been missed. Second, the compliance of patients in taking the entire bowel preparation was not reported. Therefore, we could not differentiate between insufficient bowel cleansing due to lack of compliance or to the fact that the actual protocol for bowel preparation is not sufficient for CCE. However, to our knowledge, this work is the first to provide insights on how CCE is used in daily practice and its strength and limits.
The main limitations related to CCE use are insufficient bowel cleansing and incomplete examination 20 . Despite using an optimized protocol of bowel cleansing with booster and split PEG preparation, fewer than half of CCEs were considered complete with adequate bowel cleansing, which is about 25 % less than what has been described in academic studies 21 22 . Actual strategies for bowel preparation are insufficient and new approaches should be developed 17 . Recently, Fuccio et al identified risk factors associated with poor colon cleansing for colonoscopy in hospitalized patients 23 . The systematic screening for such factors before CCE could prompt extended bowel preparation to optimize CCE diagnosis performance. However, a CCE that is incomplete or with insufficient bowel cleansing can still be of clinical interest, as demonstrated by the fact that nearly half of the CCEs with a significant polyp were described as incomplete or with insufficient bowel cleansing.
Because most missed cases of advanced neoplasia were due to incomplete CCE with distal CRC location, this raises the question of completing CCE with a distal colonoscopy in patients with contraindication to sedation and incomplete CCE. This is supported by the fact that herein, performing a distal colonoscopy after CCE would have allowed the detection of nearly all identified advanced neoplasias. Given these results, a possible recommended approach for elderly patient management would be to perform a colonoscopy after CCE in case of: 1. identification of a significant polyp; 2. insufficient bowel cleansing; or 3. identification of a polyp with an aspect suggestive of advanced malignancy; and 4. to propose only a distal colonoscopy to avoid sedation-associated risks in cases of incomplete CCE ( Fig. 3 ).
Fig. 3 .
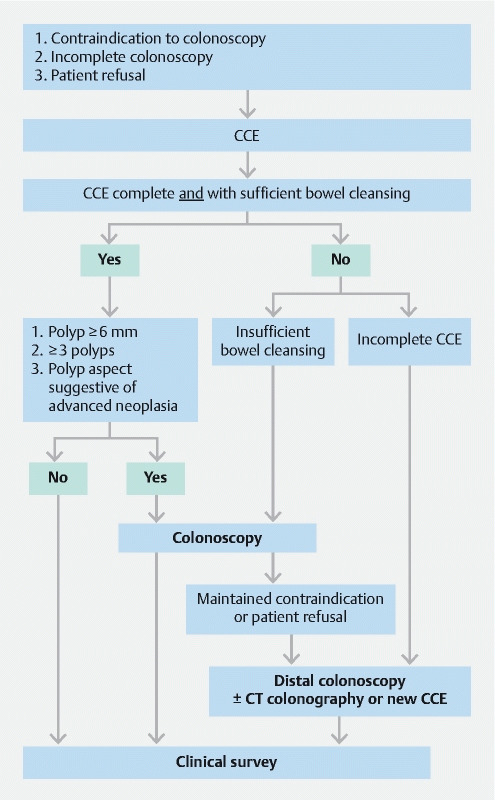
Strategy proposal for patient management according to colon capsule endoscopy (CCE) results obtained from the ONECC cohort.
Conclusions
In conclusion, the ONECC cohort showed that a complete CCE with adequate bowel preparation can be used to exclude colonic advanced neoplasia in daily practice in subjects for whom colonoscopy cannot be performed. However, improvements in completion rate and cleansing protocols are needed to enhance CCE diagnostic accuracy.
Acknowledgements
The authors would like to acknowledge all members of the ONECC Study Group : Benech N., Vinet O., Alandry G., Borotto E., Bouet C., Benamouzig R., Ponchon T., Galmiche J.P., Sacher Huvelin S., Samaha E., Saurin J.C., and V. Landel, Direction de la Recherche Clinique et de l’Innovation, Hospices Civils de Lyon for writing assistance.
Footnotes
Competing interests Dr. Benech has received a travel grant from Maat. R. Benzmouzig has received research support from Medtronic, is a consultant for Medtronic and Alfasigma, has taught for Medtronic and Mayoly Spindler, and received a Congress invitation from Mayoly Spindler. Dr. Ponchon has taught for Olympus. Dr. Dray has received research support from MSD and Norgine, done consulting for Medtronic and Boston Scientific, taught for Fujifilm, Medtronic, Alfasigma, and Bouchara-Recordati, received a Congress invitation from AbbVie, Biocodex, Boston Scientific, Hospira (groupe Pfizer), and MSD, and is a cofounder of and shareholder in Augmented Endoscopy. Dr. Sacher-Huvelin has done consulting for Medtronic. Dr. Galmiche has done consulting for Medtronic and Fujifilm. Dr. Saurin has done consulting for AbbVie, Bouchara-Recordati, Mayoly Spindler, Medtronic, ABS-Bolton, and Intromedic and received a Congress invitation from AbbVie, MSD, and Hospira (groupe Pfizer). This study was funded by Medtronic, which provided CCE, funds for e-CRF constitution, and salary for a clinical research assistant that reviewed all CCE, colonoscopy reports, and performed the follow-up.
References
- 1.Winawer S J, Zauber A G, Ho M N et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Citarda F, Tomaselli G, Capocaccia R et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–815. doi: 10.1136/gut.48.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf A MD, Fontham E TH, Church T R et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 4.Rembacken B, Hassan C, Riemann J F et al. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) Endoscopy. 2012;44:957–968. doi: 10.1055/s-0032-1325686. [DOI] [PubMed] [Google Scholar]
- 5.Eliakim R, Fireman Z, Gralnek I M et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963–970. doi: 10.1055/s-2006-944832. [DOI] [PubMed] [Google Scholar]
- 6.Spada C, Hassan C, Galmiche J P et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527–536. doi: 10.1055/s-0031-1291717. [DOI] [PubMed] [Google Scholar]
- 7.Enns R A, Hookey L, Armstrong D et al. Clinical Practice Guidelines for the Use of Video Capsule Endoscopy. Gastroenterology. 2017;152:497–514. doi: 10.1053/j.gastro.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Van Gossum A, Munoz-Navas M, Navas M M et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–270. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 9.Eliakim R, Yassin K, Niv Y et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 10.Spada C, Hassan C, Munoz-Navas M et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581–5890. doi: 10.1016/j.gie.2011.03.1125. [DOI] [PubMed] [Google Scholar]
- 11.Spada C, Pasha S F, Gross S A et al. Accuracy of first- and second-generation colon capsules in endoscopic detection of colorectal polyps: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1533–1.543E11. doi: 10.1016/j.cgh.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Rex D K, Adler S N, Aisenberg J et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148:948–95700. doi: 10.1053/j.gastro.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Saito Y, Saito S, Oka S et al. Evaluation of the clinical efficacy of colon capsule endoscopy in the detection of lesions of the colon: prospective, multicenter, open study. Gastrointest Endosc. 2015;82:861–869. doi: 10.1016/j.gie.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Sacher-Huvelin S, Coron E, Gaudric M et al. Colon capsule endoscopy vs. colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther. 2010;32:1145–1153. doi: 10.1111/j.1365-2036.2010.04458.x. [DOI] [PubMed] [Google Scholar]
- 15.Rondonotti E, Borghi C, Mandelli G et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol. 2014;12:1303–1310. doi: 10.1016/j.cgh.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Pecere S, Senore C, Hassan C et al. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest Endosc. 2020;91:406–4140. doi: 10.1016/j.gie.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Kroijer R, Dyrvig A-K, Kobaek-Larsen M et al. Booster medication to achieve capsule excretion in colon capsule endoscopy: a randomized controlled trial of three regimens. Endosc Int Open. 2018;6:E1363–E1368. doi: 10.1055/a-0732-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spada C, Hassan C, Barbaro B et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2015;64:272–281. doi: 10.1136/gutjnl-2013-306550. [DOI] [PubMed] [Google Scholar]
- 19.Utano K, Katsuki S, Matsuda T. Colon capsule endoscopy versus CT colonography in patients with large non-polypoid tumours: a multicentre prospective comparative study (4CN Study) Digestion. 2019;101:1–9. doi: 10.1159/000501609. [DOI] [PubMed] [Google Scholar]
- 20.Spada C, Riccioni M E, Hassan C et al. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol. 2011;45:119–124. doi: 10.1097/MCG.0b013e3181dac04b. [DOI] [PubMed] [Google Scholar]
- 21.Van Gossum A, Munoz-Navas M, Navas M M et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–270. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 22.Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971–977. doi: 10.1055/s-2006-944835. [DOI] [PubMed] [Google Scholar]
- 23.Fuccio L, Frazzoni L, Spada C et al. Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.02.055. [DOI] [PubMed] [Google Scholar]


