Abstract
The COVID-19 pandemic has spread rapidly across the world in 2020, affecting both adults and, to a lesser extent, children. In this article, the authors describe the neurologic manifestations of COVID-19 in children, including the epidemiology, pathogenesis, clinical features, laboratory and imaging findings, and treatment options. The management of patients with concomitant neuroimmunologic disorders and drug interactions between medications used to treat COVID-19 and other neurologic disorders (especially immune-modifying drugs) is also discussed.
Keywords: COVID-19, SARS CoV-2, Neurologic manifestations, Children
Key points
-
•
COVID-19 is predominantly a respiratory disease that affects children less commonly and less seriously than adults.
-
•
Neurologic manifestations, although more common in adults, are also being seen in children infected with the virus, especially those with multisystem inflammatory syndrome in children.
-
•
Neurologic features of the virus are highly variable, involving the central as well as the peripheral nervous system.
-
•
Multiple mechanisms of neurologic involvement by the virus have been postulated, including attachment to the neuronal ACE-2 receptors, via the olfactory nerve or immune-mediated pathogenesis. These mechanisms lead individually or collectively to an “endotheliopathy” with resultant neurologic symptoms.
-
•
No specific treatments for the central or peripheral nervous system involvement are available to date; however, caution must be advised in administering immunotherapy where required, as this may decrease the body’s innate immunity to fight the virus.
Introduction
In December 2019, a novel coronavirus, now designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported to cause a severe form of pneumonia in adults living in the Wuhan province of China in December 2019. The resultant clinical syndrome, termed COVID-19, has since been declared a pandemic, and cases have been reported from every country in the world.1 In this article, the authors describe the current state of knowledge regarding the neurologic complications of COVID-19 in children.
Epidemiology
Although children were initially thought to be immune from COVID-19, it is now known that children can indeed develop the disease and shed the virus, although a smaller proportion of the pediatric population suffers from disease-related morbidity and mortality compared with adults.2 Children form only about 1% to 5% of all COVID-19 infections worldwide, and 80% of them are either asymptomatic or have mild infection.3 In the largest published cohort thus far, 1% of affected children were less than 10 years of age and 1% were between 10 and 18 years of age.4 The youngest reported patient was a 1-day-old.5 In a study of more than 700 children from China who tested positive for the SARS-CoV-2 virus, less than 6% had severe symptoms requiring supplemental oxygen or admission to the hospital.6
In an adult cohort in Wuhan, 36.4% of patients with COVID-19 had neurologic manifestations, including headache, dizziness, stroke, or seizures.7 Neurologic manifestations were mostly noted in those with severe underlying infection, suggested by more deranged laboratory markers like lymphopenia, D-dimer, and therefore, neurologic features may suggest a higher disease burden and possibly a higher viral load. In a Chinese study of 171 children, no neurologic manifestations were reported, whereas an Italian study reported only nonspecific headaches in 4% to 28% of affected children.3 , 8 Incidence of neurologic manifestations in children with COVID-19 was reported as 9.2% in a meta-analysis of 28 pediatric studies, with a total of 199 children included.9 However, with the recently described multisystem inflammatory syndrome in children (MIS-C), which may be a postinfectious immune response to prior infection, the incidence of neurologic manifestations has reportedly increased to 34%.10
Pathophysiology
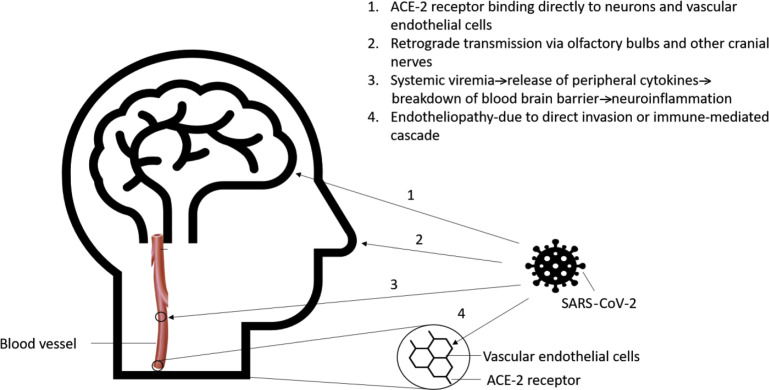
SARS-CoV-2 may enter the central nervous system (CNS) through hematogenous spread or retrograde transmission (Fig. 1 ).
Fig. 1.
Proposed mechanisms of neuroinvasion by SARS-CoV-2.
SARS-CoV-2 appears to have neurotropic potential similar to SARS-CoV and MERS (Middle East respiratory syndrome) viruses as well as other respiratory viruses, such as influenza, respiratory syncytial virus, Human Herpes Virus (HHV)-6 and -7, echovirus, and coxsackie virus. Autopsy reports of edema in the medulla oblongata with microscopic evidence of neuronal degeneration in this part of the brain stem may explain the depressed respiratory drive in infected patients.11 Indirect evidence of neurotropism with resultant astrocytic and neuronal injury is provided by studies demonstrating elevated serum levels of biomarkers such as glial fibrillary acidic protein (GFAP) and neurofilament (nFL). GFAP is a marker of glial activation, whereas nFL is indicative of neuronal injury.12 More importantly, in vitro replicability of SARS-CoV-2 has been demonstrated in pulmonary, intestinal, hepatic, renal, and neuronal cells.13
Evidence for hematogenous spread is obtained from electron microscopic studies noting the presence of viral particles in the endothelial cells of the brain capillaries.14 Angiotensin converting enzyme 2 (ACE2) has gathered interest as the binding target receptor of CoV-2 on the vascular endothelium. SARS-CoV-2 differs from SARS-CoV because of 380 amino-acid substitutions that lead to differences in the viral spike protein (S), which forms a key part of the receptor binding domain. These changes cause the novel SARS virus to have greater binding affinity with the ACE2 receptor, which is expressed on a variety of cells, including neurons.15 The entry of the virus into cells is facilitated by the interaction between the trimeric viral S protein with the extracellular domain of the transmembrane ACE2 protein. ACE2, in turn, constitutes an important mechanism for negative regulation of the renin angiotensin pathway (RAS). The disinhibition of the RAS pathway that is caused by binding of the virus to ACE2 leads to direct loss of ACE2 receptors and via proteolytic processing and shedding, may drive the systemic manifestations of COVID-19, making it a far more serious disease than SARS-CoV that primarily led to an acute respiratory distress syndrome phenotype.16
Retrograde axonal transmission predominantly via the olfactory bulb has been proposed as another potential mechanism to explain the spread of SARS-CoV-2 to the CNS. Experiments in mice have demonstrated that intranasal infection with SARS-CoV can lead to disruption of the nasal epithelium and subsequent neuroinvasion.17 The presence of anosmia in infected individuals may also lend credence to this theory of neuroinvasion via the olfactory cells, the only part of the CNS not protected by dura mater. Retrograde transmission through other cranial nerves has also been postulated via the tongue through cranial nerve VII, IX, and X to the nucleus of tractus solitarius, the thalamus, and then the cortex, or through the corneal and buccal epithelium via cranial nerve V.18
In addition to the above proposed mechanisms, the neurologic effects of SARS-CoV-2 may also be related to release of inflammatory agents that can occur with systemic viremia. These inflammatory chemicals may lead to partial break down of the blood-brain barrier (BBB), thereby allowing peripheral cytokines to gain access to the CNS, which in turn can exacerbate or trigger neuroinflammation.19 , 20 In the pediatric population, this mechanism may assume special importance as a systemic inflammatory/autoinflammatory response with multiorgan dysfunction and has been widely reported.21 , 22 Therefore, in a sense, the CNS manifestations of SARS-CoV-2 may be due to an “endotheliopathy,”-either because of direct invasion of the endothelial cells in the vasculature of the BBB or because of an immune-mediated cascade that causes swelling or inflammation of these cells.23
Clinical features
Central Nervous System
Manifestations described in children and adults
-
1.
Meningitis/encephalitis/encephalopathy: In a meta-analysis of 187 children with MIS-C, 64 (34%) were reported to have symptoms suggestive of meningitis or encephalitis, including headache, positive meningeal signs, and altered mental status. Of these children, only 8 had evaluation of the cerebrospinal fluid (CSF). Five of them showed CSF pleocytosis, but none had SARS-CoV-2 isolated via CSF reverse transcription-polymerase chain reaction (RT-PCR).10 In another study of 27 children with COVID-19 based in the United Kingdom, 4 (15%) had altered mentation, encephalopathy, headaches, and brainstem and cerebellar ataxia.24 One of the youngest reported children with COVID-19–related neurologic involvement was a 6-week-old infant who has episodes of bilateral leg stiffening and sustained upward gaze.25 Several reported cases of adults with altered mental status, meningismus, seizures, and occasionally focal neurologic deficits, including facial weakness, diplopia, and oscillopsia, have also been described.26 Some of these patients demonstrated a positive CSF RT-PCR for SARS-CoV-2.27 Apart from the neurotropism of the virus itself, another possible mechanism of the encephalopathy may be the severe hypoxia seen in these patients, which may cause cerebral vasodilation and eventually diffuse cerebral edema.28
-
2.
Seizures: In a multicenter Italian study on children with SARS-CoV-2, 3% of children were noted to have seizures, with 60% of these children having an underlying diagnosis of epilepsy.29 The seizures were not associated with fever in all cases. A detailed description of seizure semiology or duration was not provided in these cases either. In a case series of 8 critically ill pediatric patients, a 10-month-old was described who had intussusception, multiorgan dysfunction disseminated intravascular coagulation, toxic encephalopathy, and status epilepticus.30 Seizures were also reported along with fever, cough, and vomiting, in a 2-year-old girl with RT-PCR–confirmed COVID-19 by nasopharyngeal swab.31
-
3.
Stroke: Acute cerebrovascular disease in COVID-19 may manifest as intraparenchymal hemorrhage, large vessel occlusion, or venous sinus thrombosis. A 16-year-old boy with aseptic meningitis, cavernous sinus thrombosis, followed by left middle cerebral artery stroke and eventual death was described in the French literature.32 However, it is not clear if arterial strokes are predominantly due to large or small vessel disease. It is postulated that SARS-CoV-2 leads to vessel thrombosis secondary to endothelial dysfunction, stasis, platelet activation, or inflammation.33
-
4.
Possible developmental delay: Vertical transmission of the virus from mother to fetus has not been demonstrated; however, the effects of fetal exposure to chronic maternal viremia in utero on long-term neurodevelopmental outcomes are unknown at this time.18
-
5.
Loss of sense of smell and taste (anosmia and ageusia): Anosmia is commonly seen in the adults who have milder disease. A reported incidence of 34% to 89% has been noted in studies involving adults. Anosmia and ageusia have also been occasionally reported in the pediatric population.34,35 The incidence in children was estimated to be about 0.5% in a meta-analysis of 199 children.9 Another case series reported children presenting solely with anosmia and ageusia in the absence of any other manifestations of the disease.36
Manifestations described only in adults so far
-
6.
Acute disseminated encephalomyelitis (ADEM) and myelitis: Two case reports have described adults with encephalopathy and focal neurologic deficits (one with dysphagia and dysarthria; the other with seizures), normal CSF, and hyperintensities on MRI.37,38 Both patients improved with immunotherapy (steroids and intravenous immunoglobulin [IVIg], respectively). A case report from Wuhan, China described the only case of COVID and myelitis with acute flaccid paraparesis, incontinence, hyporeflexia, and a sensory level in the thoracic spine.39 MRI was not performed in this case because of high infectivity during the pandemic.
-
7.
Acute necrotizing hemorrhagic encephalopathy: A single case of symmetric, multifocal involvement of the brain parenchyma, including the thalamus, in an adult patient has been described.40 This is a known, rare entity seen postviral infections, usually in the pediatric age group and is thought to occur secondary to a cytokine storm with breakdown of BBB.
-
8.
Neuropsychiatric disorders (including neurocognitive) and dementia-like syndrome: Presenting as altered mental status, this manifestation was described in a surveillance study of 153 patients with neurologic features from the United Kingdom.41
Peripheral Nervous System
Manifestations described in children and adults
-
1.
Guillain-Barré syndrome (GBS): Several case reports have been published describing classic GBS a few days to weeks after a severe infection with SARS-CoV-2. Common presenting symptoms included lower limb/all limb weakness, paresthesia, and ataxia.42 Electrophysiological studies were consistent with demyelinating disease or axonal disease. Two such cases have also been described in children.43 , 44 These children were 11 and 15 years old, respectively, and developed weakness about 3 weeks after upper respiratory infection symptoms, and both cases had nerve conduction velocity studies consistent with GBS.
-
2.
Nonspecific peripheral nervous system: Global proximal muscle weakness and hyporeflexia were described in 15% and 7% of the children, respectively, in a small UK-based study.24
-
3.
Muscle injury and rhabdomyolysis: There have been 2 case reports of adolescents presenting with rhabdomyolysis as one of the initial symptoms, one presented with isolated rhabdomyolysis and later developed other symptoms, while the other had associated fever and shortness of breath from the onset of the disease. There has been at least 1 pediatric case of rhabdomyolysis albeit a 16 year old.45 Cases with rhabdomyolysis with elevated creatine kinase resulting from muscle injury have also been described in adults as a late manifestation of COVID-19.46 , 47
Aside from the clinical manifestations of the disease itself, it should also be recognized that the features and impact of the disease may be different in patients with underlying comorbidities. Patients with neuromuscular disease should be considered at a higher risk of complications, because of possible involvement of breathing and swallowing muscles.48 Infection with SARS-CoV-2 may also cause exacerbation or progression of underlying myasthenia gravis and spinal muscular atrophy.49 A French study described a 17-year-old girl with underlying severe neonatal encephalopathy and epilepsy, who presented with severe respiratory distress secondary to COVID-19. The decision to withdraw care and not intubate was made because of underlying severe comorbidities and poor quality of life.32
Investigations
MRI of the Brain
MRI of the brain in children with neurologic manifestations of COVID-19 has been reported to demonstrate acute lesions in the splenium of the corpus callosum.24 Such lesions may be representative of intra-myelin edema and as such may occur in a variety of disorders, including other forms of viral encephalitis, demyelination, or CNS malignancies, such as lymphoma.50 Larger case series in adults who have involvement of the CNS in COVID-19 point to the presence of acute/subacute infarcts, leukoencephalopathy involving the subcortical and deep white matter, cortical abnormalities on fluid-attenuated inversion recovery sequences, microhemorrhages, and leptomeningeal enhancement as the more common features. Posterior reversible encephalopathy syndrome–like features, acute hemorrhagic necrotizing encephalopathy, and dural venous sinus thrombosis were less frequently reported, and only 15% patients had normal imaging.51 However, because large case series of this nature are lacking in children, it cannot be stated with certainty if similar findings are likely to be present in the pediatric age group. It is possible that certain radiological findings may only be present in adults with COVID-19. For instance, although a temporary increase in the size of the olfactory bulbs, signal changes, and contrast uptake has been reported in certain adults with COVID-19–related anosmia, no such changes could be documented in children with anosmia.52 A few reports commenting on the use of other imaging modalities, such as PET, are emerging in adults but not yet in the pediatric age group.53
Electroencephalography
EEG features of COVID-19 appear to be nonspecific and consist of generalized slowing.24
Cerebrospinal Fluid Studies
CSF has been reported to be acellular in most reported cases, with negative SARS-CoV-2 cultures and no other evidence of inflammatory disease process, such as presence of oligoclonal bands. RT-PCR presence of SARS-CoV-2 in CSF has been reported to be positive in rare cases of adults with encephalitis, but no such cases have been reported yet in children.42
Histopathology
Histopathological correlates are now emerging, and cases with ADEM-like pathologic condition are being reported with axonal injury, perivascular lymphocytic infiltration, neuronal loss, and interstitial inflammatory changes.54
Treatment options
Treatment of Neurologic Complications of COVID-19
No specific treatment exists for the neurologic manifestations, and the treating physician must depend on symptom-based management. IVIg and plasmapheresis have been used in the treatment of ADEM and GBS, as they are in non-COVID–based situations. Antiepileptics must be used for the management of seizures. Critically ill children may require management of airway and circulation issues in a critical care unit.
Treatment of Other Neurologic Conditions During the COVID-19 Pandemic
Medications used for migraines in pediatric patients are considered safe for use during the pandemic, with the initial reservations regarding the use of nonsteroidal anti-inflammatory drugs, including ibuprofen, having been dismissed by the World Health Organization and European Medicines Agency.55 , 56
Treatment of Patients with Underlying Neuroimmunologic Disorders
An important consideration is the treatment of patients with underlying neuroimmunologic diseases, including multiple sclerosis and neuromyelitis optica spectrum disorder. It is not entirely clear if these patients are at a higher risk of severe COVID-19 infection and complications compared with the general population and depending on the specific medications they are on. Alemtuzumab, cladribine, fingolimod, and B-cell–depleting agents (used in individuals with multiple sclerosis) may increase risk of infection, whereas glatiramer, dimethyl fumarate, and teriflunomide should not.45 In general, it has been proposed that steroids, IVIg, and plasmapheresis are safe to administer during the pandemic in the case of an exacerbation of the underlying condition. Patients who are already on immunosuppressive therapy should continue the same therapy during the pandemic and even if they develop mild symptoms. Those with a severe COVID-19 infection may need to stop immunosuppressants, but this is best determined based on the specific situation and underlying factors.
Drug Interactions Between COVID-19 Therapy and Drugs Used in Neurologic Conditions
Treatments for the COVID-19 disease are constantly evolving, and several medications have been suggested and studied. In general, they include anti-inflammatory agents (usually corticosteroids), antivirals (remdesivir, favipiravir), and in most cases, anticoagulation drugs (usually aspirin or clopidogrel).57 Other medications, like hydroxychloroquine and ivermectin, were also suggested at the beginning of the pandemic, but their efficacy in this infection is not well proven. Some patients on chloroquine have been shown to have seizures.58 Chloroquine and hydroxychloroquine have also been shown to prolong QTc and should be used with caution in patients on tricyclic antidepressants (used in children with migraine or depression), which have the same side effect.
Hydroxychloroquine, chloroquine, and remdesivir carry the risk of drug interactions and enzymatic induction/inhibition when combined with carbamazepine, phenytoin, phenobarbital, and primidone, which are commonly used antiepileptic agents.28 Ritonavir, also used in some cases, can lead to induction of CYP450.59 Azithromycin should be used with caution in patients with myasthenia gravis, as it may exacerbate symptoms or cause new-onset myasthenia gravis.60 Tocilizumab and anakinra have not been associated with any CNS side effects or interactions with any drugs. Patients with preexisting neurologic conditions requiring infliximab can generally be continued without significant adverse effects or increased risk for COVID-19. However, enough data are not available, and registries are required to collect more information.1
Differential diagnosis
There is an extensive list of differential diagnoses for the neurologic complications of the virus. It includes several other respiratory viruses, as well as noninfectious causes that may be associated with each symptom. It is important, however, to consider COVID-19 or a COVID-19 postinflammatory syndrome, such as MIS-C, when evaluating a patient with any of the manifestations described above. It is important to note that the sensitivity and specificity of the testing process are not 100%, leading to possible missed diagnoses. In addition, case reports of different and novel findings are being published on a daily basis.
Summary
The COVID-19 pandemic has affected upwards of 93 million people worldwide, causing more than 2 million deaths so far (as of January 15, 2021). It has affected adults more than children; however, the disease affects people of different age groups in a dissimilar manner, resulting in protean manifestations. The proportion of children with CNS and peripheral nervous system involvement in those infected with COVID-19 may be low; however, the sheer number of patients infected with the virus could make the absolute number of patients with these complications exceptionally large. Therefore, continued collection of accurate data, detailed descriptions of various neurologic manifestations, along with efforts to isolate the virus from CSF and autopsy samples will provide more answers in the future. Up until that point, management decisions will have to be made according to available evidence on a case-by-case basis.
Clinics care points
-
•
Management of neurologic complications of COVID-19 is similar to that of non-COVID-19–affected patients, including intravenous immunoglobulin and plasmapheresis for inflammatory demyelination and antiepileptic drugs for seizures.
-
•
Medications for migraines are generally considered to be safe. There are no limitations to using nonsteroidal anti-inflammatory drugs in severe acute respiratory syndrome coronavirus 2–infected patients.
-
•
In those with underlying neuroimmunologic conditions, steroids, intravenous immunoglobulins, and plasmapheresis are safe to administer during the pandemic in the case of an exacerbation of the underlying condition. Patients who are already on immunosuppressive therapy should continue the same therapy during the pandemic and even if they develop mild symptoms of COVID-related disease. Those with severe COVID-19 infection may need to temporarily suspend immunosuppressants, but this is best determined based on the specific situation and underlying factors.
Footnotes
The authors have no financial relationships to disclose.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christy A. COVID-19: a review for the pediatric neurologist. J Child Neurol. 2020;35(13):934–939. doi: 10.1177/0883073820939387. [DOI] [PubMed] [Google Scholar]
- 3.Parri N., Lenge M., Buonsenso D. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz N., Treptow A., Schmidt S., et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J. 2020;39(8):e212. doi: 10.1097/INF.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pousa P.A., Mendonça T.S.C., Oliveira E.A., et al. Extrapulmonary manifestations of COVID-19 in children: a comprehensive review and pathophysiological considerations. J Pediatr (Rio J). 2020;97(2):116–139. doi: 10.1016/j.jped.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T.H. Neurological involvement associated with COVID-19 infection in children. J Neurol Sci. 2020;418:117096. doi: 10.1016/j.jns.2020.117096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanberg N., Ashton N.J., Andersson L.M., et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 13.Chu H., Chan J.F., Yuen T.T., et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paniz-Mondolfi A., Bryce C., Grimes Z., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92(7):699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gheblawi M., Wang K., Viveiros A., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desforges M., Le Coupanec A., Dubeau P., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafstrom C.E., Jantzie L.L. COVID-19: neurological considerations in neonates and children. Children (Basel) 2020;7(9):133. doi: 10.3390/children7090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt M.P., Bolding K.A., Wayne C.R., et al. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of postinfectious autoimmune encephalitis. Proc Natl Acad Sci U S A. 2020;117(12):6708–6716. doi: 10.1073/pnas.1911097117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cain M.D., Salimi H., Diamond M.S., et al. Mechanisms of pathogen invasion into the central nervous system. Neuron. 2019;103(5):771–783. doi: 10.1016/j.neuron.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Nakra N.A., Blumberg D.A., Herrera-Guerra A., et al. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushik S., Aydin S.I., Derespina K.R., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Mannan O., Eyre M., Löbel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugue R., Cay-Martínez K.C., Thakur K.T., et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94(24):1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong P.F., Craik S., Newman P., et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med (Lond) 2020;20(3):293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsini A., Corsi M., Santangelo A., et al. Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients. Neurol Sci. 2020;41(9):2353–2366. doi: 10.1007/s10072-020-04544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garazzino S., Montagnani C., Donà D., et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25(18):2000600. doi: 10.2807/1560-7917.ES.2020.25.18.2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D., Li H., Lu X.X., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16(3):251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X., Huang J., Zhao F., et al. [Clinical features of children with SARS-CoV-2 infection: an analysis of 13 cases from Changsha, China] Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(4):294–298. doi: 10.7499/j.issn.1008-8830.2003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oualha M., Bendavid M., Berteloot L., et al. Severe and fatal forms of COVID-19 in children. Arch Pediatr. 2020;27(5):235–238. doi: 10.1016/j.arcped.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomelli A., Pezzati L., Conti F., et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mak P.Q., Chung K.-S., Wong J.S.-C., et al. Anosmia and ageusia: not an uncommon presentation of COVID-19 infection in children and adolescents. Pediatr Infect Dis J. 2020;39(8):e199–e200. doi: 10.1097/INF.0000000000002718. [DOI] [PubMed] [Google Scholar]
- 37.Zanin L., Saraceno G., Panciani P.P., et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T., Hirsh E., Zandieh S., et al. COVID-19-Associated acute multi-infarct encephalopathy in an asymptomatic CADASIL patient. Neurocritical care. 2020:1–4. doi: 10.1007/s12028-020-01119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao K., Huang J., Dai D., et al. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv. 2020 [Epub ahead of print] [Google Scholar]
- 40.Poyiadji N., Shahin G., Noujaim D., et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varatharaj A., Thomas N., Ellul M.A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalifa M., Zakaria F., Ragab Y., et al. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatr Infect Dis Soc. 2020;9(4):510–513. doi: 10.1093/jpids/piaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank C.H.M., Almeida T.V.R., Marques E.A., et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. 2020 doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korsukewitz C., Reddel S.W., Bar-Or A., et al. Neurological immunotherapy in the era of COVID-19 - looking for consensus in the literature. Nat Rev Neurol. 2020;16(9):493–505. doi: 10.1038/s41582-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(7):1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suwanwongse K., Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12(4):e7561. doi: 10.7759/cureus.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guidon A.C., Amato A.A. COVID-19 and neuromuscular disorders. Neurology. 2020;94(22):959–969. doi: 10.1212/WNL.0000000000009566. [DOI] [PubMed] [Google Scholar]
- 49.Gummi R.R., Kukulka N.A., Deroche C.B., et al. Factors associated with acute exacerbations of myasthenia gravis. Muscle Nerve. 2019;60(6):693–699. doi: 10.1002/mus.26689. [DOI] [PubMed] [Google Scholar]
- 50.Doherty M.J., Jayadev S., Watson N.F., et al. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005;62(3):433–437. doi: 10.1001/archneur.62.3.433. [DOI] [PubMed] [Google Scholar]
- 51.Gulko E., Oleksk M.L., Gomes W., et al. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature review. AJNR Am J Neuroradiol. 2020;41(12):2199–2203. doi: 10.3174/ajnr.A6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatipoglu N., Mine Yazici Z., Palabiyik F., et al. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia in pediatric cases. Int J Pediatr Otorhinolaryngol. 2020;139:110469. doi: 10.1016/j.ijporl.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delorme C., Paccoud O., Kas A., et al. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur J Neurol. 2020;27(12):2651–2657. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szperka C.L., Ailani J., Barmherzig R., et al. Migraine care in the era of COVID-19: clinical pearls and plea to insurers. Headache. 2020;60(5):833–842. doi: 10.1111/head.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agency E.M. European Medicines Agency; The Netherlands: 2020. EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19. [Google Scholar]
- 57.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mülhauser P., Allemann Y., Regamey C. Chloroquine and nonconvulsive status epilepticus. Ann Intern Med. 1995;123(1):76–77. doi: 10.7326/0003-4819-123-1-199507010-00021. [DOI] [PubMed] [Google Scholar]
- 59.Brooks J., Daily J., Schwamm L. Protease inhibitors and anticonvulsants. AIDS Clin Care. 1997;9(11):87–90. [PubMed] [Google Scholar]
- 60.May E.F., Calvert P.C. Aggravation of myasthenia gravis by erythromycin. Ann Neurol. 1990;28(4):577–579. doi: 10.1002/ana.410280417. [DOI] [PubMed] [Google Scholar]