Abstract
Background
Invasive fungal infections (IFI) are increasing in prevalence in recent years. In the last few months, the rise of COVID-19 patients has generated a new escalation in patients presenting opportunistic mycoses, mainly by Aspergillus. Candida infections are not being reported yet.
Objectives
We aimed to determine the prevalence of systemic candidiasis in patients admitted to ICUs due to severe pneumonia secondary to SARS-CoV-2 infection and the existence of possible associated risk factors that led these patients to develop candidiasis.
Patients/methods
We designed a study including patients with a confirmed diagnosis of COVID-19.
Results
The prevalence of systemic candidiasis was 14.4%, and the main isolated species were C. albicans and C. parapsilosis. All patients that were tested positive for Candida spp. stayed longer in the ICU in comparison to patients who tested negative. Patients with candidiasis had higher MuLBSTA score and mortality rates and a worse radiological involvement. In our study, Candida spp. isolates were found in patients that were submitted to: tocilizumab, tocilizumab plus systemic steroids, interferon type 1β and Lopinavir-Ritonavir.
Conclusions
Results suggested a high prevalence of systemic candidiasis in severe COVID-19-associated pneumonia patients. Patients with Candidiasis had the worst clinical outcomes. Treatment with tocilizumab could potentialize the risk to develop systemic candidiasis.
Keywords: Candida, Systemic candidiasis, COVID-19, Viral pneumonia
1. Introduction
Invasive fungal infections (IFI) have an incidence of approximately 4.7 per 1,000 patients and are associated with high morbidity and mortality in critically ill individuals. Approximately 90% of IFI deaths are caused by Candida spp., Aspergillus spp., Cryptococcus spp. and Pneumocystis spp [1]. Although studies indicate an increase in IFI, its incidence is underestimated [2]. Evidence shows that IFI and Influenza (A or B) co-infections occur in approximately 32% of immunocompromised and in 14% of immunocompetent patients [3].
For COVID-19, the reported incidence of IFIs ranged between 4% and 27.7% [4,5] according to the studies consulted. In addition, the associated risk factors for the development of these infections are not fully known. Nevertheless, some authors have been shown that treatment with systemic corticosteroids alone or associated with other immunomodulating drugs was associated with the development of IA in COVID-19 patients [6]. Another factor associated with the development of Invasive Aspergiloses (IA) is lymphopenia. In severe COVID-19, like in Influenza, patients could develop lymphopenia [7]. Frequently, seriously ill COVID-19 patients, who develop an adult respiratory distress syndrome (ARDS), are admitted to Intensive Care Units (ICU) where the invasive monitoring can allow for the entry of opportunistic pathogens. To make matters worse, these patients may also receive immunosuppressive (IS) medication, such as systemic corticosteroids (SC), tocilizumab (TCZ) and cyclosporine (CP) that are, on one hand, fundamental to halt the “cytokine storm” that occurs in the most severe cases [8,9] but on the other hand can potentially increase the susceptibility of these patients to co-infections. Cases of severe pulmonary fungal infection in patients treated with TCZ, such as mucormycosis, allergic bronchopulmonary aspergillosis and pulmonary Pasteurellosis, have been described [[10], [11], [12]].
Invasive candidiasis, mostly candidemia, is associated with a high global mortality rate, ranging from 36% to 63% in different patients’ groups [13]. In a prospective hospital-based population study in seven European countries, rates of candidemia ranging from 0.20 to 0.38 per 1,000 hospital admissions were reported [14]. Approximately half of all Candida infection occurs in ICU [15], represented 5–10% of all ICU-acquired infections, with high mortality in the range of 40% [16]. Although it is described high critically ill patients colonized with Candida spp., only 5–40% develop an invasive infection [17]. In the last decades, many risk factors associated with IC development have been identified (diabetes mellitus, renal failure, urinary or vascular catheters, neutropenia, immunosuppression, major surgery, neutropenia, burns, among others) [18]. IFI, especially IA, are well-known complications of influenza [19]. Aspergillus isolation in influenza patients is associated with high mortality, but it is unclear what role Aspergillus plays in this process [20].
To date, case series studies of invasive aspergillosis, but not of candidiasis, have been reported in patients with COVID-19. With that said, the main hypothesis of this work was that the use of IS drugs increases the risk of systemic Candida spp. co-infection in patients with severe SARS-CoV-2. Based on that, the major aim was to determine the prevalence of systemic candidiasis in patients admitted to ICUs due to severe pneumonia secondary to SARS-CoV-2 infection (COVID-19-Associated Candidiasis, CAC) and the existence of possible associated risk factors that led these patients to develop candidiasis.
2. Methods
2.1. Study design
We designed an observational prospective study that was conducted in the Rey Juan Carlos University Hospital (HURJ, https://www.hospitalreyjuancarlos.es), from February 1st to April 30th, 2020. HURJ is a center integrated into the Madrid (Spain) public health system providing healthcare to approximately 174,000 people in the southern area of Madrid. Under normal circumstances, this center has 358 individual hospital beds and can double the amount, if necessary, e.g., due to the surge of COVID-19 cases at the Pneumology department. The Pneumology department has a Respiratory Intermediate Care Unit (RICU), with four beds, which is integrated into the Intensive Care Unit (ICU). During the COVID-19 pandemic, the ICU and RICU were unified, reaching up to 45 hospital beds, and the number of intensive care beds was increased overall.
The present study was approved by the Ethics Committee of the Fundación Jiménez Díaz Health Research Institute (EO102-20-HRJC). In view of the pandemic situation, informed consent was not requested from the patients. Personal information and data obtained from the subjects were kept confidential.
2.2. Sample design
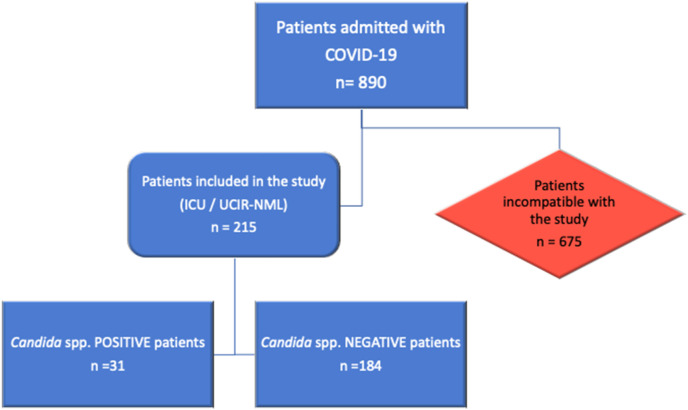
A total of 215 patients (>18 years old) were admitted to RICU or ICU, with a confirmed diagnosis of severe pneumonia caused by SARS-CoV-2 [6], with or without ARDS. To confirm the presence of systemic candidiasis in these patients (in our study, invasive candidiasis comprises candidemia and deep-seated candidiasis, which may occur concurrently or independently), we combined multiple techniques to improve the global sensitivity of all of them. In this study, we used the following diagnostic-test: culture tests for Candida spp. (blood culture and culture of invasive samples) and nonculture diagnostic test including mannan and anti-mannan IgG test, C. albicans germ tube antibody, BDG and PCR-based assays, (Fig. 1 ). We followed the bedside scoring system reported by the Spanish group (EPCAN Study). This “Candida score” allows early antifungal treatment when candidemia is suspected in non-neutropenic ICU patients [7,8].
Fig. 1.
Flow chart of patients and samples included in the study. From the 890 COVID-19 patients that were admitted to the Rey Juan Carlos University Hospital (HURJ), from February 1st to April 30th, 2020, 215 individuals needed ICU/RICU support. Among those, 31 patients were Candida spp.-positive (14.419%) and the rest (85.581%) were negative for this fungal pathogen.
Patients that were tested negative for mycosis or SARS-CoV-2 infection and those from whom the data was not collected were excluded from this study.
To confirm the presence of SARS-CoV-2 infection, smear samples were collected from the upper respiratory tract, at the moment of admission, and processed for RT-PCR (“Primerdesign Ltd COVID-19 genesig® Real-Time PCR assay”, HAIN Lifescience, Chandler's Ford, UK). The serological diagnosis was made using the COVID-19 rapid Biozek Medical Test (specificity 98% for IgG and 96% for IgM; sensitivity 100% and 85% respectively, BIOZEK, Apeldoorn, Netherlands).
2.3. Microbiological diagnosis
We used criteria to invasive Candidiasis published by Clancy CJ et al. [9]. During the pandemic situation, invasive Candidiasis diagnostic was made by culture test, relegating serum assays for Candida antigens and anti-Candida antibodies to isolated cases. We assume that without the no-culture test we would have had underdiagnosed cases of invasive candidiasis. Therefore, we consider that this fact does not distort the results presented.
According to our protocol, every two weeks all patients admitted to the RIC-ICU or ICU had samples collected from respiratory secretions or from nasal, oropharyngeal, rectal, urinary, and skin exudates. If Candida spp. was isolated in these cultures, studies to rule out invasive candidiasis were carried out through the culture of sterile specimens [10].
Blood cultures are positive in patients with deep-seated candidiasis (only 50–70% of cases). In those patients, cultures of infected tissues were performed [11]. Similarly, to the tests described previously, the tips removed from the central catheters were sent to the microbiology laboratory for further analysis. For fungal diagnosis, samples were cultivated in CHROMagar Candida Medium (CHROMagar Company, Paris, France) and identified by AuxaColor™ 2 (Bio-rad, Marnes-la-Coquette, France) and API Candida systems (bioMerieux, France).
For the diagnosis of invasive pulmonary candidiasis, we obtained invasive samples of respiratory secretions by fiberoptic bronchoscopy. Fiberoptic bronchoscopy (Pentax™, Tokyo, Japan) was performed to obtain additional samples from respiratory secretions via direct aspiration (bronchoaspiration, BAS) or through a bronchoalveolar lavage (BAL). To perform the BAL, three sterile 50 mL physiological saline solution aliquots (0.85% Sodium Chloride solution, 150 mL total) were instilled in the area where radiological involvement was detected. The test was prescribed and performed by the same pulmonologist who did the fibrobronchoscopy.
2.4. Variables
Patient data was obtained from the electronic medical record system, which allows for access to complete medical, nursing, laboratory, and radiology information.
The patient data was analyzed considering several variables, such as socio-demographic characteristics, age, sex, need for admission, associated comorbidities, previous immunosuppressive treatment, administration of COVID-19 treatment and the patient evolution during admission (complication rate, number of days the patient was hospitalized and death rate).
Patients were scored as severe if they had pneumonia with organ failure (one or more organs), <90% oxygen saturation (measured via pulse oximetry in ambient air) or respiratory rate >30 breaths per minute (BPM). For ARDS cases, the scoring was made according to PaO2/FiO2 ratios (PaO2, Partial Pressure of Oxygen and FiO2, Fraction of inspired oxygen) that were either calculated or corrected from SpO2/FiO2 (SpO2, arterial oxygen saturation). The score was set as mild (200 mmHg < PaO2/FiO2 < 300 mmHg), moderate (100 mmHg < PaO2/FiO2 < 200 mmHg) or severe (PaO2/FiO2 < 100 mmHg) [12].
The reference for the severity index used here was the Multi-lobular infiltrates (5 points), Lymphocyte count ≤0.8 × 109/L (4 points), Bacterial coinfection (4 points, presented with bacteria positive by laboratory tests or sputum tests and there were consolidation signs on CT feature), acute Smoker (3 points, and the patients who had quit-smoking history were scaled as 2 points), hyperTension (2 points), and Age ≥ 60 years (2 points) score (MuLBSTA). It presented better specificity and sensitivity than CURB-65 as a predictor of mortality in viral pneumonias. Twelve points were considered to be the threshold for the MuLBSTA analysis to differentiate between the low-risk (0–11 points) and the high-risk mortality groups (≥12 points) [21].
2.5. Statistical analysis
Data were analyzed using SPSS statistical software version 20.0 (SPSS Inc., Chicago, IL, USA). Continuous variables with normal distribution were expressed either as means ± standard deviation or as medians. Qualitative and categorical variables were reported as numbers and percentages, respectively. Statistical differences between groups were analyzed using the exact Chi-square (χ2) and/or Fisher's exact test. The calculation of relative risk (RR) was made using contingency tables and the results were expressed as a confidence interval (CI). Differences between pair groups were analyzed using the unpaired Student's T-test or the Mann-Whitney U test, as appropriate. Pearson's bivariate correlation test was used as an independent predictor. Groups were considered statistically different when p < 0.05.
3. Results
A total number of 215 patients were tested and Candida spp. was identified in 31 individuals (14.4%) from 41 biological samples that were isolated. Most of the species identified were C. albicans (n = 23) and C. parapsilosis (n = 10) obtained from urine samples or respiratory secretions (Table 1 ). C. glabrata, C. dubliniensis, C. krusei and C. tropicalis were also found in COVID-19-positive patients (Table 1).
Table 1.
Summary of which Candida spp. species were isolated from different patients’ samples.
| Clinical isolate | Urine | BAL | Central catheter | Blood culture | Total |
|---|---|---|---|---|---|
| C. albicans (n) | 7 | 12 | 3 | 1 | 23 |
| C. dubliniensis (n) | 2 | 1 | 0 | 0 | 3 |
| C. glabrata (n) | 3 | 0 | 0 | 0 | 3 |
| C. krusei (n) | 1 | 0 | 0 | 0 | 1 |
| C. parapsilosis (n) | 4 | 4 | 0 | 2 | 10 |
| C. tropicalis (n) | 1 | 0 | 0 | 0 | 1 |
| Σ = | 18 | 17 | 3 | 3 | 41 |
n: number of isolates; = total.
There were no significant differences between patients with and without Candida spp. in terms of age (62 vs. 65 years, p-value = 0.125), sex (p-value = 0.368), or the presence of comorbidities (Charlson index 2.06 vs 3.41, p-value = 0.103). Regarding the latter, no differences were found with respect to the prevalence of certain chronic diseases, such as obstructive pulmonary disease (COPD) (p-value = 0.419), asthma (p-value = 0.224), type 2 diabetes mellitus (p-value = 0.719), or previous immunosuppressive treatment. However, statistical differences were found concerning the presence of malignancies (9 solid organ malignancies and 2 Hodgkin lymphomas) in patients with Candida spp. (p-value = 0.0001).
All Candida spp. positive patients were admitted to the ICU, required orotracheal intubation, received treatment with parenteral nutrition and had nasogastric or bladder catheters and central access routes. The time that these patients spent in ICU were longer in comparison with COVID-19 patients that did not have Candida spp. co-infection (21.7 vs 12.1 days, p-value = 0.001). In addition, Candida spp. positive patients had a higher MuLBSTA pneumonia severity index (12.13 vs. 10.08, p-value = 0.000002). All Candida spp.-positive patients developed ARDS (7 moderate and 11 severe cases), with a SpO2/FiO2 of 79.18, at the time of admission, whereas the group without candidiasis had a ratio of 127.29 (p-value = 0.009). Moreover, Candida spp.-positive individuals showed a more extensive radiological involvement at the time of admission (93% presented severe involvement vs. 60% without Candida spp., p-value = 0.001), a higher rate of complications during their stay in the ICU (p-value = 0.009) and higher mortality rates (87% Candida spp.-positive group vs. 36% Candida spp.–negative group, p-value = 0.000008).
Regarding treatment, when the patients with or without the Candida spp. were compared, there was a statistically significant association between the presence of Candida spp. and certain monotherapy treatments prescribed, such as TCZ (Relative Risk (RR), 1.378, IC95% (0.94–2.03); p-value = 0.05) or TCZ plus methylprednisolone or dexamethasone (SC) (p-value = 0.010). Similarly, patients who received treatment with interferon type 1β (IFN-1β) (RR 1.382; IC95% (0.81–62.5); p-value = 0.049) or with Lopinavir-Ritonavir (LPV-RTV) (RR 1.709, IC95% (1.11–2.62); p-value = 0.002) (Table 2 ) were also tested positive for candidiasis.
Table 2.
Pharmacological treatment of COVID-19 patients and differences between patients with or without Candida spp. isolation.
| Treatment regimens | Candida spp.- positive group (n = 31) | Candida spp.-negative group (n = 120) | p-value | Relative Risk (RR) |
|---|---|---|---|---|
| Antibiotics prescription | ||||
| Azithromycin; n (%) | 28 (90.3) | 112 (93.3) | 0.565 | |
| Cephalosporine; n (%) | 30 (96.7) | 118 (98.3) | ||
| Fluoroquinolone; n (%) | 5 (16.1) | 30 (25) | ||
| Carbapenems; n (%) | 3 (9.6) | 10 (8.3) | ||
| Aminoglycosides; n (%) | 2 (6.5) | 7 (5.8) | ||
| Vancomicine; n (%) | 1 (3.2) | 5 (4.2) | ||
| Others; n (%) | 0 | 20 (16.7) | ||
| Hydroxychloroquine; n (%) | 31 (100) | 118 (98.3) | 0.469 | |
| Lopinavir-Ritonavir; n (%) | 18 (58.1) | 34 (28.3) | 0.002 | 1.709, IC95% (1.11–2.62) |
| Tocilizumab; n (%) | 16 (51.6) | 40 (33.3) | 0.05 | 1.378, IC95% (0.94–2.03) |
| Total dose (mg) | 546 | 544 | ||
| Corticosteroids; n (%) | 27 (87.1) | 108 (90) | 0.640 | 1.033 |
| Starting dose (mg) | 140 | 123 | ||
| Treatment regimen; n (%) | ||||
| Regimen 1 | 2 (11.7) | 43 (17) | ||
| Regimen 2 | 10 (58.8) | 113 (45) | 0.138 | |
| Regimen 3 | 5 (29.4) | 96 (38) | ||
| Cyclosporine; n (%) | 5 (16.1) | 11 (9.2) | 0.262 | 1.083 |
| Interferon type 1β; n (%) | 9 (32.1) | 1 (6.2) | 0.049 | 1.382, IC95% (0.81–62.5) |
| Combined IS treatment: | 54,8% | 36% | ||
| TCZ + SC | 12 (38.7) | 43 (28.7) | 0.010 | |
| TCZ + CP | 0 | 0 | ||
| SC + CP | 0 | 7 (4.7) | ||
| TCZ + SC + CZ | 5 (16.1) | 10 (6.7) |
Corticosteroid treatment: Guideline 1 Boluses of 125 mg of methylprednisolone (MTP); Guideline Boluses of 125 mg of MTP, followed by a guideline of 0.5–1 mg/kg/ideal weight for 10 days: Guideline 3 MTP 0.5–1 mg/kg/ideal weight for 10 days. IS: Immunosuppressants TCZ: Tocilizumab SC: Systemic corticosteroids CP: Cyclosporine.
We compared the Candida group with another mycosis isolated. In our series, Aspergillus spp. was isolated in 7 cases, and Trichosporon asahii in one case. Main differences between Candida spp. and Aspergillus spp. shows in Table 3 . No differences in clinical, radiological involvement or gas exchanges were found, except lower SpO2/FiO2 rate in Candida spp. group (79.18 vs 123.7, p-value = 0.04) and more frequent treatment with corticosteroids in patients which Candida spp. was isolated vs patients with Aspergillus spp. (87.1% vs 51.1%, p-value = 0.05).
Table 3.
Summary table about the differences between Candida spp. and Aspergillus spp. groups.
| Candida spp. (n = 23) | Aspergillus spp. (n = 7) | p-value | |
|---|---|---|---|
| Age (±SD) | 62 (7.1) | 60.5 (14) | 0.560 |
| Sex (M:F) | 62.5:37.5 | 71.4:28.6 | 0.664 |
| Frail Index (±SD) | 1.56 (0.53) | 0.5 (0.7) | 0.254 |
| Charlson index (±SD) | 2.06 (4) | 2 (2.8) | 0.079 |
| MuLBSTA (±SD) | 12.13 (1.6) | 10 (4.2) | 0.693 |
| CURB-65 (±SD) | 1.60 (0.55) | 0.5 (0.7) | 0.074 |
| Lymphocytes (±SD) | 750 (3,162) | 1,500 (6,393) | 0,337 |
| C-protein reactive (±SD) | 17.5 (6.7) | 29 (15.6) | 0.481 |
| Dimer-D (±SD) | 1,669 (2,807) | 505 (558) | 0.595 |
| LDH (±SD) | 500 (210) | 473 (70) | 0.869 |
| Ferritin (±SD) | 1,549 (1,295) | 1,664 (1,725) | 0.918 |
| PaO2/FiO2 (±SD) | 123 (43.5) | 136.4 (118) | 0.598 |
| SpO2/FiO2 (±SD) | 79.18 (21) | 123.7 (64) | 0.04 |
| HACOR index (±SD) | 5.71 (1.9) | 4 (5.6) | 0.742 |
| ROX index (±SD) | 3.4 (1) | 4 (2.7) | 0.804 |
| ARDS classification: | |||
| n (%) | |||
| Moderate | 7 (30) | 1 (14.3) | 0.150 |
| Severe | 11 (70) | 6 (85.7) | |
| Radiological involvement: | |||
| n (%) | |||
| Mild | 0 | 0 | |
| Moderate | 2 (7) | 1 (14.3) | 0.218 |
| Severe | 21 (93) | 6 (85.7) | |
| Immunosuppressants drugs: | |||
| n (%) | |||
| Tocilizumab | 16 (51.6) | 5 (71.4) | 0.846 |
| Cyclosporine | 5 (16.1) | 1 (14.2) | 0.430 |
| Corticosteroids | 27 (87.1) | 4 (57.1) | 0.05 |
| OTI n (%) | 23 (100) | 7 (100) | 1 |
| Days admission (±SD) | 31.6 (7.2) | 32.3 (14) | 0.212 |
| ICU days admission (±SD) | 21.7 (11.5) | 27.3 (12.4) | 0.818 |
| Death n (%) | 20 (87) | 6 (86) | 0.950 |
Differences between the clinical, radiological, gas exchange and severity findings of COVID-19 were analyzed. SD: Standard deviation LDH: lactate dehydrogenase ARDS: Adult respiratory distress syndrome ICU: Intensive Care Unit OTI: orotracheal intubation.
4. Discussion
Opportunistic mycoses are a serious complication of viral co-infections. Candida spp. are major constituents of the human mycobiome and the main cause of invasive fungal infections in a critical care setting (about 6–10% patients) with high mortality (19–40% of patients with invasive candidiasis) [22]. It was reported that approximately 20–30% of patients admitted to the ICU with severe influenza A H1N1 also had invasive aspergillosis. IFIs have been playing an important role also in COVID-19 patients [20,23]. To date, this is the first study that investigates the occurrence of IFIs due to Candida spp. and the associated risk factors in patients suffering from severe pneumonia due to SARS-CoV-2 (COVID-19-Associated Candidiasis, CAC). There is one case study that has been reported in which a COVID-19-positive female patient had skin manifestations due to a C. parapsilosis co-infection [24]. In the present work, we found that Candida spp. was identified in 14.4% of COVID-19-positive patients. This occurrence was higher than the 4% fraction that had been previously reported in patients affected by other viral pneumonias [25]. This result suggests that the real incidence of mycosis in COVID-19 patients is probably higher than what is being reported. The main isolated species reported here were C. albicans followed by C. parapsilosis, mostly in BAL samples. However, other species, such as C. glabrata, C. dubliniensis, C. krusei and C. tropicalis were also found in COVID-19 patients. We also investigated patients with candiduria for confirmation of associated invasive candidiasis or candidemia. According to Bougnoux et al., candiduria has a higher risk of developing candidemia [26,27]. In our study, 18 patients had candiduria and, in only three cases, they confirmed the existence of concomitant invasive candidiasis (16.7%).
Candida spp.-positive group had a greater severity of pneumonia, defined by a higher score on the MuLBSTA index (p-value < 0.001), higher degree of radiological involvement (p-value = 0.001) and lower mean PaO2/FiO2 values (p-value = 0.009). All patients needed to be admitted to the ICU where they stayed longer than patients without Candida spp. (21.7 vs 12.1 days, p-value = 0.001). In addition, Candida spp.-positive patients had a higher rate of complications during admission (p-value = 0.009) and a higher mortality rate (p-value <0.001). This data is consistent with what has been described in terms of candidiasis occurrence in patients suffering from other viral pneumonias [25]. CAC group was indeed the group with more severe patients. According to our results, these patients presented severe ARDS, with a lower PaO2/FiO2 ratio, extensive radiological involvement, and patients received more immunosuppressants drugs for all these reasons. Moreover, lymphocytes counts were lower than patients without candidiasis. Therefore, we thought that the severe clinical illness could be related to CAC, which we assumed was related to COVID-19, which delayed the CAC diagnostic.
According to the data presented here, treatment with TCZ alone or in combination with SC, IFN-1β or LPV-RTV increased the risk of systemic candidiasis (p-value = 0.05; 0.010; 0.049 and 0.002, respectively). IFN-1β is a cytokine with antiviral and immunoregulatory capacity. Its role in fungal infections is controversial, although it could have a “protective effect” [28]. However, the precise effect of IFN-1β on the host defense against Candida spp. is still under debate [29]. Various IFN-1β gene polymorphisms modulate Candida-induced cytokine production. This implicates in an increase of TNF-α, IL-1β and IL-10 levels and a decrease in IL-6 and IL-8 which increases the overall susceptibility to systemic candidiasis [30]. According to our data the RR was 1,382 (IC95% (0.81–62.5)). Currently, the treatment with IFN-1β is not indicated for COVID-19 patients due to the increased risk of mortality from hepatitis and renal failure [31].
TCZ is a monoclonal antibody against soluble IL-6 receptors which has been approved for the treatment of refractory rheumatoid arthritis (RA). In patients with severe COVID-19, a cytokine-release syndrome occurs, especially of IL-6, and it is triggered by the viral infection itself [32]. For this reason, TCZ is used as a treatment option. The use of TCZ in RA has been associated with an increased risk of bacterial and fungal infections since the blockage of IL-6 interferes directly with the proliferation of B lymphocytes and with the differentiation and cytotoxicity of T lymphocytes (LT) [33]. Several studies have shown the role of IL-6 during C. albicans infection. In vitro experiments showed that Candida cell wall components stimulate IL-6 production [34,35]. IL-6 −/− murine models suggest that these animals are more prone to develop Candida infections probably due to an impaired neutrophil function in the absence of this cytokine [36]. Van Enckevort et at., reported a 90% mortality rate in IL-6 −/− mice vs 40% in the control group after infusion of 105 CFU C. albicans. The increased mortality was related to the increase in the number of C. albicans found in the liver and kidney of the IL-6 −/− mice [28]. Isolated cases of systemic mycosis have also been published in RA patients treated with TCZ [[37], [38], [39]].
The RR of candidemia in patients treated with LPV-RTV was 1,709, IC95% (1.11–2.62). Heretofore, mycosis cases in patients treated with LPV-RTV have been associated with an immune reconstruction syndrome and an exaggerated secondary inflammatory response [40,41]. In severely immunosuppressed HIV patients, the improvement in LTCD4+ function led to an increase in the response to mitogens and a change in the cytokine pattern towards the TH-1 response, with an increase in IFN-γ and IL-2 production. It is currently unclear whether the cytokine release syndrome is the result of an exaggerated release of inflammatory cytokines or due to a loss of immune regulation and production of regulatory cytokines [42]. There is no current data regarding the role of LPV-RTV in the pro-inflammatory status of patients with COVID-19. Probably, in the gravest cases with severe lymphopenia (χ = 733/mm3, SD ± 300/mm3), the improvement in the count and function of LTCD4 enhances the cytokine deregulation which favours infection by opportunistic mycoses such as candidiasis. Targeted studies are necessary to understand the immune pattern of the BAL isolated from the most severe COVID-19 patients to interpret the effects of the drugs used for treating these patients and the susceptibility to infection with Candida spp.
There were no significant differences according to the type of fungus isolated. It is true that patients with Candida had a lower SpO2/FiO2 ratio and received a higher proportion of treatment with oral corticosteroids. However, in both groups, the fungal infection darkened the prognosis. All patients were admitted to the ICU and needed OTI. Overall, patients with IFI associated high mortality, 86% Aspergillus group vs. 87% Candida group (p-value = 0.950).
In the present investigation, some bias must be considered. Our study was conducted during the SARS-CoV-2 outbreak, with a collapse of the healthcare system. It is a real-life study without a defined control group. We assume the existence of a delay in the diagnosis and treatment of patients with systemic candidiasis, which could impact the prognosis. Furthermore, the extensive areas of lung parenchymal involvement by ARDS could mask other lesions suggestive of IFI. Patients with CAC received immunosuppressive treatment more frequently than the control group. The CAC group indeed had more criteria for severity, although we cannot differentiate for sure whether the highest severity was defined by COVID-19 or by an undiagnosed Candida infection early. Despite the limitations, we consider that our results reflect a real situation experienced in most countries and with future applicability.
In conclusion, the opportunistic Candida spp. the infection rate in patients with severe COVID-19 is much higher than what previously described. The main result presented here was the increased risk of infection by Candida spp. in COVID-19 patients treated with TCZ, IFN-1β and LPV-RTV. This data corroborates the need to increase scrutiny on severe COVID-19 patients that receive immunosuppressants in order to test them for candidiasis. In these cases, COVID-19 treatment combined with specific antifungals against Candida spp. might be necessary to increase the chances of patient survival.
Ethics declarations
The present study was approved by the Ethics Committee of the Fundación Jiménez Díaz Health Research Institute (EO102-20-HRJC). In view of the pandemic situation, informed consent was not requested from the patients. Personal information and data obtained from the subjects were kept confidential.
Transparency declaration
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Gonzalo Segrelles-Calvo: Conceptualization, are responsable for the conception and design of the study, Funding acquisition, or acquisition of data, Formal analysis, Data curation, or analysis and interpretation of data, Writing – original draft, drafting the article and revising it critically for important intellectual content. Glauber R. de S Araújo: Conceptualization, are responsable for the conception and design of the study, Funding acquisition, or acquisition of data, Formal analysis, Data curation, or analysis and interpretation of data, Writing – original draft, drafting the article and revising it critically for important intellectual content. Estefanía Llopis-Pastor: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Javier Carrillo: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Marta Hernández-Hernández: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Laura Rey: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Nestor Rodríguez Melean: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Inés Escribano: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Esther Antón: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Celia Zamarro: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Mercedes García-Salmones: Funding acquisition, Formal analysis, Data curation, are responsable for the acquisition of data and analysis and interpretation of data. Susana Frases: Conceptualization, are responsable for the conception and design of the study, Funding acquisition, or acquisition of data, Formal analysis, Data curation, or analysis and interpretation of data, Writing – original draft, drafting the article and revising it critically for important intellectual content.
Acknowledgements
This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001 and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
References
- 1.Brown G.D., Denning D.W., Gow N.A.R., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004404. 165rv13-165rv13. [DOI] [PubMed] [Google Scholar]
- 2.Das R. An overview of changing trends in systemic fungal infections. WebmedCentral Microbiol. 2012;3:1–8. https://www.webmedcentral.com/wmcpdf/Article_WMC003386.pdf [Google Scholar]
- 3.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., Lagrou K., Verweij P.E., Van de Veerdonk F.L., Gommers D., Spronk P., Bergmans D.C.J.J., Hoedemaekers A., Andrinopoulou E.-R., van den Berg C.H.S.B., Juffermans N.P., Hodiamont C.J., Vonk A.G., Depuydt P., Boelens J., Wauters J. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel Coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.England J.T., Abdulla A., Biggs C.M., Lee A.Y.Y., Hay K.A., Hoiland R.L., Wellington C.L., Sekhon M., Jamal S., Shojania K., Chen L.Y.C. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2021;45:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pemán J., Zaragoza R. Current diagnostic approaches to invasive candidiasis in critical care settings. Mycoses. 2010;53:424–433. doi: 10.1111/j.1439-0507.2009.01732.x. [DOI] [PubMed] [Google Scholar]
- 8.León C., Ruiz-Santana S., Saavedra P., Almirante B., Nolla-Salas J., Alvarez-Lerma F., Garnacho-Montero J., León M.A. EPCAN Study Group, A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit. Care Med. 2006;34:730–737. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]
- 9.Clancy C.J., Nguyen M.H. Diagnosing invasive candidiasis. J. Clin. Microbiol. 2018;56 doi: 10.1128/JCM.01909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García J., Pemán J. Microbiological diagnosis of invasive mycosis. Rev. Iberoam. De. Micol. 2018;35:179–185. doi: 10.1016/j.riam.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Clancy C.J., Nguyen M.H. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013;56:1284–1292. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 12.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S., ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin Definition. J. Am. Med. Assoc. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Kullberg B.J., Verweij P.E., Akova M., Arendrup M.C., Bille J., Calandra T., Cuenca-Estrella M., Herbrecht R., Jacobs F., Kalin M., Kibbler C.C., Lortholary O., Martino P., Meis J.F., Muñoz P., Odds F.C., De Pauw B.E., Rex J.H., Roilides E., Rogers T.R., Ruhnke M., Ullmann A.J., Uzun Ö., Vandewoude K., Vincent J.-L., Donnelly J.P. European expert opinion on the management of invasive candidiasis in adults. Clin. Microbiol. Infect. 2011;17:1–12. doi: 10.1111/j.1469-0691.2011.03615.x. [DOI] [PubMed] [Google Scholar]
- 14.Tortorano A.M., Peman J., Bernhardt H., Klingspor L., Kibbler C.C., Faure O., Biraghi E., Canton E., Zimmermann K., Seaton S., Grillot R. Epidemiology of candidaemia in europe: results of 28-month European confederation of medical mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:317–322. doi: 10.1007/s10096-004-1103-y. [DOI] [PubMed] [Google Scholar]
- 15.Bassetti M., Righi E., Costa A., Fasce R., Molinari M.P., Rosso R., Pallavicini F.B., Viscoli C. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect. Dis. 2006;6:21. doi: 10.1186/1471-2334-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glöckner A., Karthaus M. Current aspects of invasive candidiasis and aspergillosis in adult intensive care patients. Mycoses. 2011;54:420–433. doi: 10.1111/j.1439-0507.2010.01885.x. [DOI] [PubMed] [Google Scholar]
- 17.Garbino J., Lew D.P., Romand J.-A., Hugonnet S., Auckenthaler R., Pittet D. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002;28:1708–1717. doi: 10.1007/s00134-002-1540-y. [DOI] [PubMed] [Google Scholar]
- 18.Eggimann P., Barberini L., Calandra T., Marchetti O. Invasive Candida infections in the ICU. Mycoses. 2012;55:65–72. doi: 10.1111/j.1439-0507.2011.02151.x. [DOI] [Google Scholar]
- 19.Abbott J.D., Fernando H.V.J., Gurling K., Meade B.W. Pulmonary Aspergillosis following post-influenzal bronchopneumonia treated with antibiotics. BMJ. 1952;1:523–525. doi: 10.1136/bmj.1.4757.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30237-X. e48–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M., Qu J. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arastehfar A., Carvalho A., Nguyen M.H., Hedayati M.T., Netea M.G., Perlin D.S., Hoenigl M. COVID-19-Associated candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J. Fungi. 2020;6:211. doi: 10.3390/jof6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., Hallek M., Jung N., Klein F., Persigehl T., Rybniker J., Kochanek M., Böll B., Shimabukuro‐Vornhagen A. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz R.A., Kapila R. Cutaneous Manifestations of a 21st Century Worldwide fungal epidemic possibly complicating the COVID-19 pandemic to jointly menace mankind. Dermatol. Ther. 2020 doi: 10.1111/dth.13481. [DOI] [PubMed] [Google Scholar]
- 25.Antinori S., Milazzo L., Sollima S., Galli M., Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur. J. Intern. Med. 2016;34:21–28. doi: 10.1016/j.ejim.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Bougnoux M.-E., Kac G., Aegerter P., D'Enfert C., Fagon J.-Y., CandiRea Study Group Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med. 2008;34:292–299. doi: 10.1007/s00134-007-0865-y. [DOI] [PubMed] [Google Scholar]
- 27.He Z., Su C., Bi Y., Cheng Y., Lei D., Wang F. Evaluation of a novel laboratory candiduria screening protocol in the Intensive Care Unit. Infect. Drug Resist. 2021;14:489–496. doi: 10.2147/IDR.S289885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNab F., Mayer-Barber K., Sher A., Wack A., O'Garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeekens S.P., Ng A., Kumar V., Johnson M.D., Plantinga T.S., Van Diemen C., Arts P., Verwiel E.T.P., Gresnigt M.S., Fransen K., Van Sommeren S., Oosting M., Cheng S.C., Joosten L.A.B., Hoischen A., Kullberg B.J., Scott W.K., Perfect J.R., Van Der Meer J.W.M., Wijmenga C., Netea M.G., Xavier R.J. Functional genomics identifies type i interferon pathway as central for host defense against Candida albicans. Nat. Commun. 2013;4 doi: 10.1038/ncomms2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Vidal C., Salavert Lletí M. Immunopathogenesis of invasive mould infections. Rev. Iberoam. De. Micol. 2014;31:219–228. doi: 10.1016/j.riam.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Nacif L.S., Zanini L.Y., Waisberg D.R., Pinheiro R.S., Galvão F., Andraus W., D'Albuquerque L.C. COVID-19 in solid organ transplantation patients: a systematic review. Clinics. 2020;75 doi: 10.6061/clinics/2020/e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X., Liang Y., Ding X., Tan G., Tang S., Liu L., Liu Y., Pan Y., Wang Z. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. 2020;69:599–606. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimoto N., Kishimoto T. Interleukin 6: from bench to bedside. Nat. Clin. Pract. Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 34.Castro M., Bjoraker J.A., Rohrbach M.S., Limper A.H. Candida albicans induces the release of inflammatory mediators from human peripheral blood monocytes. Inflammation. 1996;20:107–122. doi: 10.1007/BF01487749. [DOI] [PubMed] [Google Scholar]
- 35.Steinshamn S., Waage A. Tumor necrosis factor and interleukin-6 in Candida albicans infection in normal and granulocytopenic mice. Infect. Immun. 1992;60:4003–4008. doi: 10.1128/iai.60.10.4003-4008.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Enckevort F.H.J., Netea M.G., Hermus A.R.M.M., Sweep C.G.J., Meis J.F.G.M., Van Der Meer J.W.M., Jan Kullberg B. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice 1, Med. Mycol. 1999;37:419–426. doi: 10.1046/j.1365-280X.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 37.Uchida T., Okamoto M., Fujikawa K., Yoshikawa D., Mizokami A., Mihara T., Kondo A., Ohba K., Kurohama K., Nakashima M., Sekine I., Nakamura S., Miyazaki Y., Kawakami A. Gastric mucormycosis complicated by a gastropleural fistula: a case report and review of the literature. Med. (United States). 2019;98 doi: 10.1097/MD.0000000000018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda H., Kida H., Yoshida M., Tomita T., Fujii M., Ihara S., Goya S., Tachibana I., Kawase I. Recurrent allergic bronchopulmonary aspergillosis in a patient with rheumatoid arthritis treated with etanercept and Tocilizumab. Mod. Rheumatol. 2011;21:660–664. doi: 10.1007/s10165-011-0449-0. [DOI] [PubMed] [Google Scholar]
- 39.Gastaldi R., Sicaud A., Gaudin P., Baillet A. Pulmonary Pasteurellosis in a patient treated with Tocilizumab for rheumatoid arthritis. J. Clin. Rheumatol. 2017;23:451–452. doi: 10.1097/RHU.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 40.Otto S.B.J., George P.E., Mercedes R., Nabukeera-Barungi N. Cryptococcal meningitis and immune reconstitution inflammatory syndrome in a pediatric patient with HIV after switching to second line antiretroviral therapy: a case report. BMC Infect. Dis. 2020;20 doi: 10.1186/s12879-020-4797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oosten A.W., Sprenger H.G., Van Leeuwen J.T.M., Meessen N.E.L., Van Assen S. Bilateral renal aspergillosis in a patient with AIDS: a case report and review of reported cases. AIDS Patient Care STDS. 2008;22:1–5. doi: 10.1089/apc.2007.0051. [DOI] [PubMed] [Google Scholar]
- 42.Price P., Mathiot N., Krueger R., Stone S., Keane N.M., French M.A. Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy. 2001. www.elsevier.com/locate/jcv [DOI] [PubMed]