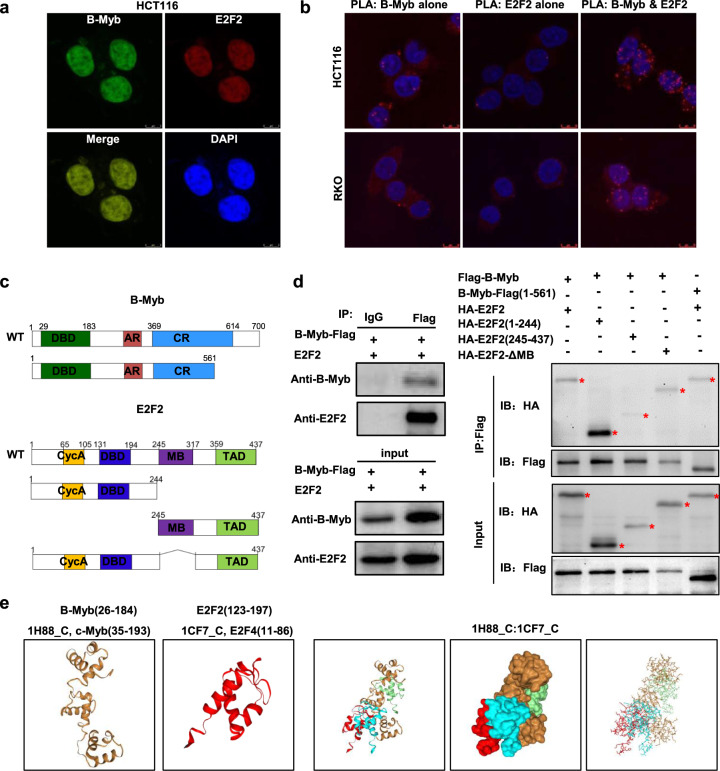
Fig. 7. Interaction between E2F2 and B-Myb.
a Colocalization of B-Myb and E2F2 protein expression in cell nuclei. HCT116 cells were co-transfected with LV203-B-Myb-Flag and pcDNA3.0-E2F2 expression vector. Forty-eight hours after the transfection, cells were fixed and stained with anti-B-Myb antibody (green) and anti-E2F2 antibody (red). Scale bar 5 μm. b Proximity ligation assays (PLAs) of B-Myb with E2F2 in HCT116 and RKO cells. Red spots are regions of signal amplification. Nuclear stain (Hoechst) is blue. Incubation with either B-Myb or E2F2 antibody alone was used as a control. Scale Bars: 10 μm. c Schematic illustration of the B-Myb and E2F2 structure and deletion mutants. DBD DNA-binding domain, AR acidic region/transactivation domain, CR conserved region, Cyc A Cyclin A/CDK2 binding domain, MB marked box, TAD Transactivation domain. d B-Myb associates with E2F2. HCT116 cells were co-transfected with LV203-B-Myb-Flag and pcDNA3.0-E2F2 expression vector (left panel). HEK293 cells were transiently co-transfected with LV105-Flag-B-Myb or pCDH-puro-HA-E2F2 with the indicated deletion mutants. Forty-eight hours after the transfection, whole cell lysates were prepared and subjected to co-immunoprecipatation assay. e Predicted models of B-Myb-E2F2 protein docking. The 3D homologous structures of B-Myb and E2F2 were searched by online HDOCK server, and the three-dimensional homologous docking models for B-Myb and E2F2 interaction were predicted.