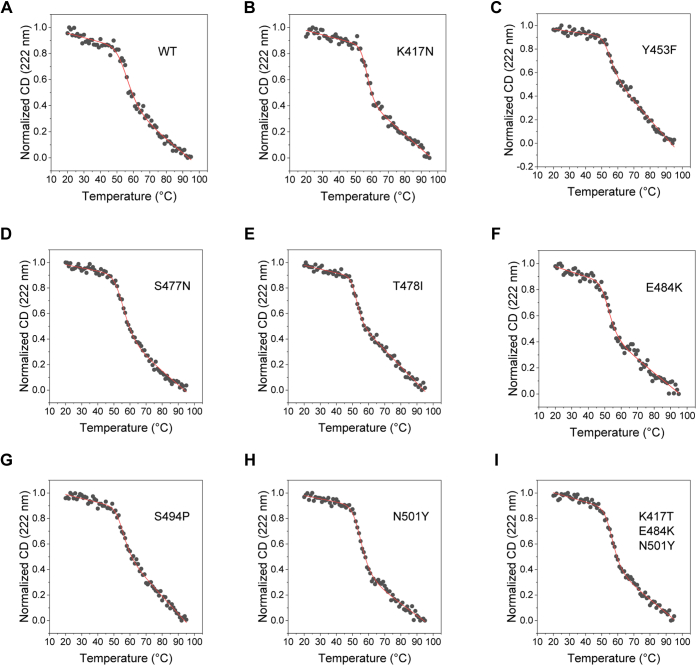
Figure 4.
Thermal denaturation melts of RBD and its mutants obtained using far-UV CD spectroscopy.A–I show the data for the wildtype RBD, single amino-acid mutations K417N, Y453F, S477N, T478I, E484K, S494P, N501Y, and for the triple mutant K417T/E483K/N501Y, respectively. The solid lines show the fits to a two-state unfolding equation (Equation 1 in the Experimental procedures section). Table 2 lists the Tm (midpoint melting temperature) and the ΔH (enthalpy change at Tm) values of RBD variants. RBD, receptor-binding domain.