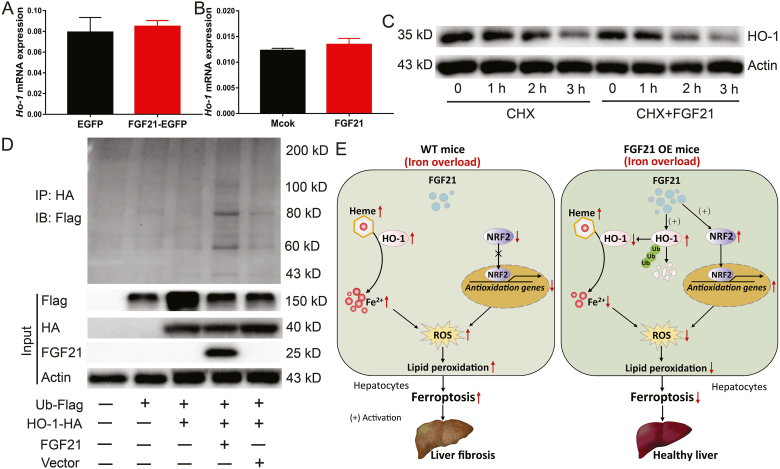
Fig. 7.
FGF21 attenuates iron overload-induced ferroptosis through promoting HO-1 ubiquitination.
(A) Ho-1 mRNA expression levels in the liver of FGF21 over-expression mice (n = 10). (B) Ho-1 mRNA expression levels in primary hepatocytes treated with recombinant FGF21 protein (n = 4). (C) HO-1 protein expression in primary hepatocytes treated with PBS (left) or 200 ng/ml recombinant FGF21 protein (right) for 4 h, followed by 20 μg/ml CHX for 3 h. (D) HEK293T cells were transfected with Flag-tagged ubiquitin cDNA (Ub-Flag) alone or together with HA-tagged Ho-1 cDNA (HO-1-HA) alone or together with FGF21 cDNA or vector plasmid for 36 h. Cell lysates were subjected to immunoprecipitation with anti-HA antibody, and the tag of flag was detected by western blot. (E) Model of proposed mechanism of FGF21 attenuated liver injury and fibrosis induced by iron overload. Iron overload induces hepatocyte ferroptosis, which contributes to liver injury and fibrosis in WT mice (Left panel). Mechanistically, iron overload robustly increases HO-1 expression, which catalyzes the conversion of heme into Fe2+, carbon monoxide and biliverdin. Notably, excess Fe2+ accumulating in the cells induces ROS generation which could be the direct reason for ferroptosis. In addition, iron overload inhibits the expression of NRF2, which is a master regulator of the antioxidant response. Of particular interest, FGF21 over expression (OE) attenuates liver injury and fibrosis induced by iron overload via suppressing hepatocytes ferroptosis (right panel). FGF21 not only enhances HO-1 ubiquitination and degradation to reduce Fe2+ generation in hepatocytes but also activates NRF2 expression to suppress iron overload-induced oxidative damage. Therefore, FGF21 is a novel regulator of ferroptosis and activation of FGF21 may provide an effective strategy for potential treatment in iron-mediated ferroptosis-related disease, such as HH.