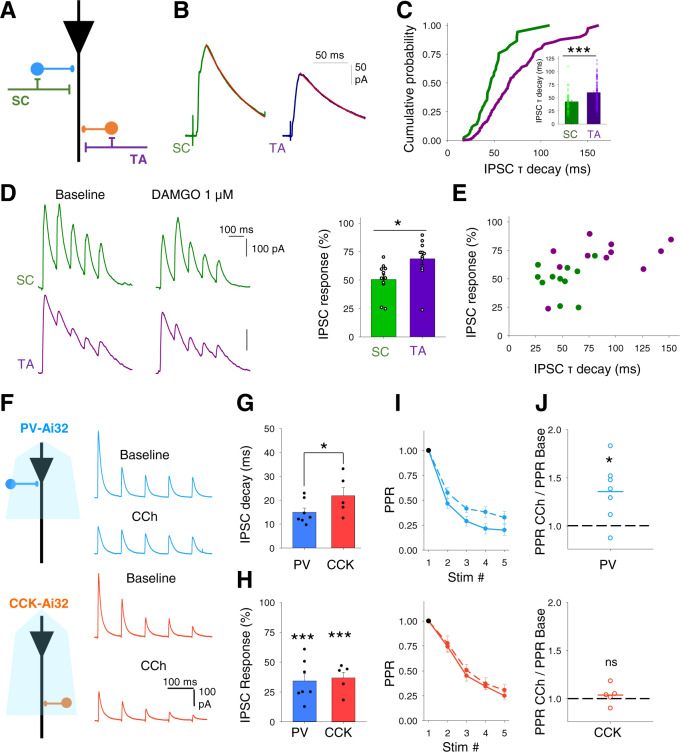
Fig. 3. Cholinergic modulation of inhibitory inputs from distinct feedforward interneuron populations.
A Schematic representation of different feedforward interneuron populations engaged by Schaffer collateral (SC) and temporoammonic (TA) pathways within CA1. B, C Disynaptic feedforward IPSCs (B) and distribution of decay kinetics (C) for Schaffer collateral (SC, green) and temporoammonic (TA, purple) input pathways demonstrating distinct populations of feedforward interneurons. Quantification of the IPSC tau decay (insert C) (SC, n = 45 from 24 mice; TA, n = 92 from 36 mice; p = 0.0002). D µ-opioid receptor agonist DAMGO (1 µM) depression of disynaptic feedforward IPSCs from SC and TA pathways (SC vs TA pathways, n = 11 from 4 mice, p = 0.015). E IPSC decay kinetics and sensitivity to DAMGO correlate and distinguish SC from TA evoked IPSCs. F Optogenetic activation of either PV (top) or CCK (bottom) interneurons at 10 Hz evoked a train of IPSCs in CA1 pyramidal neurons. IPSCs from both interneurons are depressed by CCh (10 µM). G, H IPSCs from PV interneurons display faster decay kinetics than IPSCs from CCK interneurons (G PV, n = 7; CCK, n = 5; p = 0.02) but CCh depressed the IPSC amplitudes of the first responses in the train by a similar amount (H PV, n = 7, p < 0.0001; CCK, n = 5, p < 0.0001). I, J IPSCs from both PV and CCK interneurons demonstrated frequency-dependent depression. Frequency-dependent depression was reduced after CCh application for PV (top; n = 7, p = 0.010) but not CCK (bottom; n = 5; p = 0.172) evoked IPSCs. Data are mean ± SEM; Comparisons are two-tailed paired t-test ***p < 0.001, **p < 0.01, *p < 0.05. Source data are provided as a Source Data file.