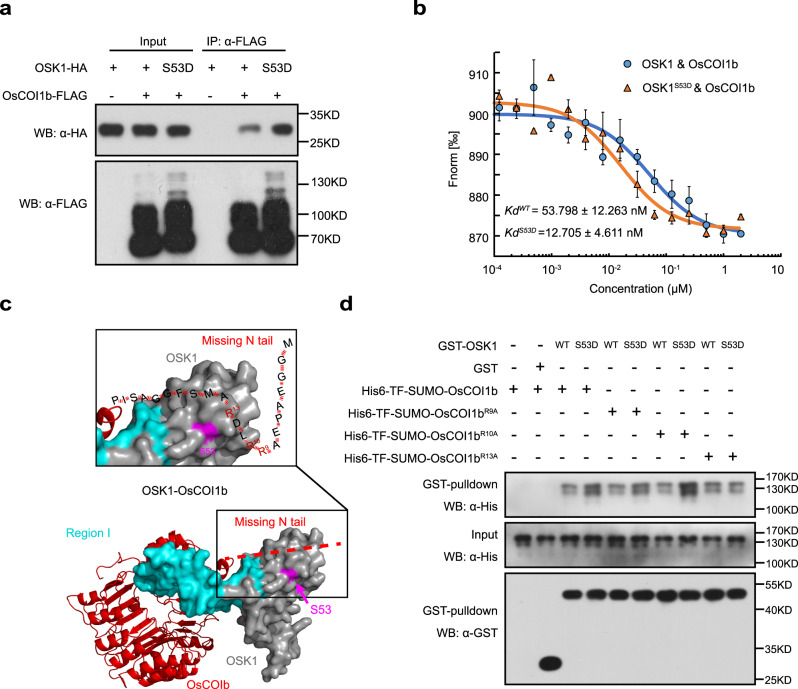
Fig. 6. The enhanced binding affinity of phosphomimic OSK1S53D to COI1b is dependent on the Arg13 residue in COI1b.
a OSK1S53D-HA bound to OsCOI1b-FLAG much stronger than OSK1-HA in co-IP assays. OSK1-HA and OSK1S53D-HA were individually co-expressed with OsCOI1b-FLAG in rice protoplasts. The immunocomplex and input proteins were analyzed by immunoblotting using anti-HA and anti-FLAG antibodies. The experiments were independently repeated 3 times with similar results. b His6-OSK1S53D bound to OsCOI1b much stronger than His6-OSK1 in MST assays. OsCOI1b bound to His6-OSK1S53D with a Kd of 12.705 ± 4.611 nM while to GST-OSK1 with a Kd of 53.798 ± 12.263 nM. Data are shown as means ± SE (n = 3 technical replicates). c The 3-D structure of OsCOI1b-OSK1 was constructed via homology modeling with the ASK1-COI1-JAZ complex (PDB: 3OGM) as a template. The N-terminal flexible tail of OsCOI1b missing from the template was arbitrarily labeled. The N-terminal tail containing multiple positive-charged Arg residues is predicted to be adjacent to Ser53 in OSK1. d OsCOI1bR13A had a similar binding affinity to OSK1 and OSK1S53D. In vitro-purified His6-TF-SUMO-tagged OsCOI1bR9A, OsCOI1bR10A and OsCOI1bR13A were individually incubated with GST-OSK1 and GST-OSK1S53D, respectively. After GST pulldown assays, the input and pulldown were detected with anti-His and anti-GST antibodies. The experiments were independently repeated 3 times with similar results.