Figure 2.
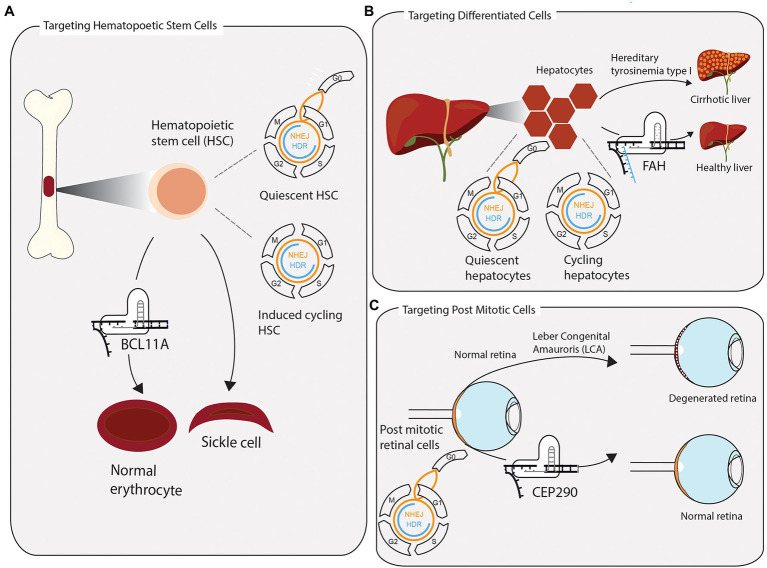
Successful clustered regularly interspaced short palindromic repeats (CRISPR) applications require consideration of tissue-specific DNA repair and repair pathway accessibility. (A) Hematopoietic stem cells (HSCs) are extracted from the bone marrow and edited ex vivo for the treatment of sickle cell anaemia and β-thalassemia. Without stimulating cells to enter the cell cycle once before CRISPR-Cas9 editing, quiescent HSCs rely on error prone NHEJ to repair induced DSB. In a clinical application, the preference for NHEJ is leveraged to disrupt the transcription factor BCL11A, which represses the expression of foetal hemoglobin. The re-expression of foetal hemoglobin allows for the formation of normally shaped erythrocytes. (B) The toxic accumulation of fumarylacetoacetate in fatal hereditary tyrosinemia type I (HTI) leads to liver cirrhosis and liver failure due to a mutation in the fumarylacetoacetate hydrolase gene (FAH). Highly differentiated quiescent cells can be stimulated to re-enter the cell cycle upon DNA, or tissue, damage. Provided with a single-stranded repair template, few cycling hepatocytes have access to repair DSB via homologous directed repair. Precisely edited hepatocytes have a growth advantage over non-edited cells and reconstitute tissue homeostasis. (C) Leber congenital amauroris (LCA) is the first disease treated with an in vivo CRISPR approach. The post mitotic light sensitive cells in the retina degenerate with age, leading to impaired vision early on in life. Appropriating the propensity of post mitotic cells to repair DSBs via NHEJ, the therapy aims to disrupt an aberrant splicing site in exon 26 of CEP290, maintaining a functional retina.