SCHEME 3.
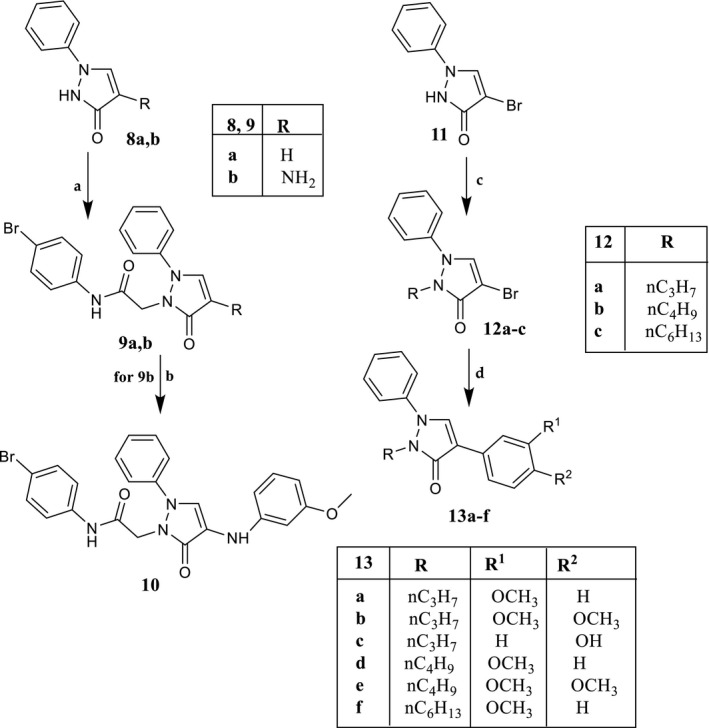
Reagents and conditions: (a) N‐(4‐bromophenyl)‐2‐chloroacetamide, K2CO3, anhydrous CH3CN, reflux, 2–7 hr. (b) For 9b: 3‐methoxybenzeneboronic acid, (CH3COO)2Cu, Et3N, activated molecular sieves, anhydrous CH2Cl2, rt, 3 hr. (c) Alkyl bromide, K2CO3, anhydrous DMF, reflux, 1 hr. (d) Tetrakis(triphenylphosphine)palladium(0), arylboronic acid, Na2CO3, anhydrous toluene, rt, 15–18 hr; for compounds 13b,c and 13e,f, reflux, 6–12 hr
