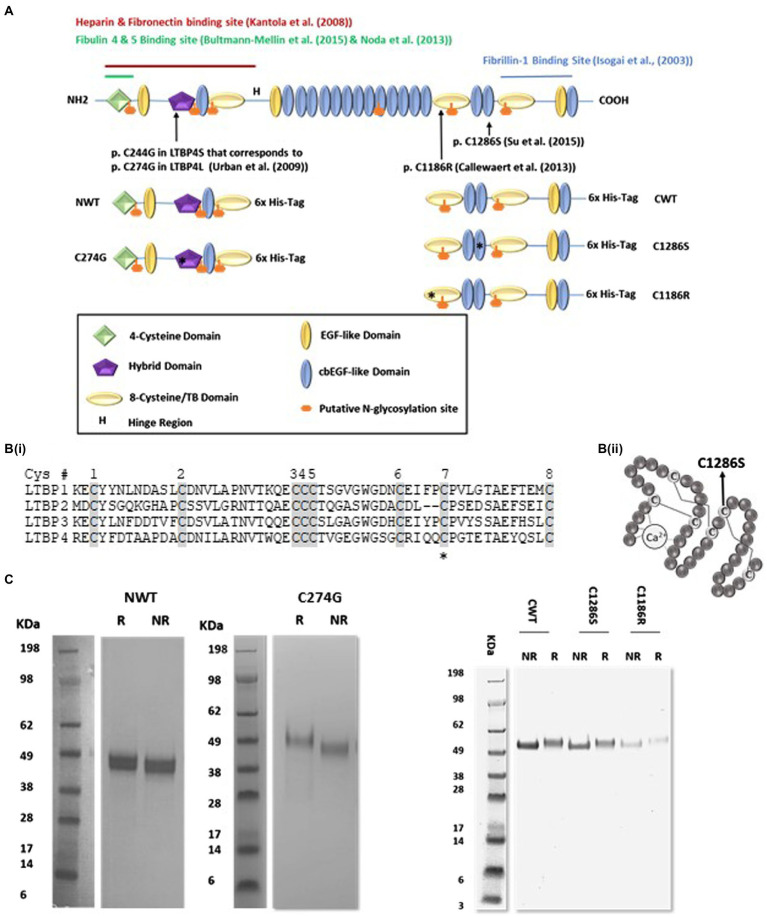
Figure 1.
Expression and purification of recombinant latent TGFβ binding protein-4 (LTBP4). (A) Schematic diagram of the domain organisation of full-length human LTBP4S with interaction sites indicated and the LTBP4 constructs with 6x-His-tag used in this study. Arrows and asterisks indicate the positions of the ARCL1C mutations. (B) (i) Amino acid sequence of the second TB domain in LTBP1-4 with the eight conserved cysteine residues shaded. C1186 is the C7 residue (highlighted with an asterisk), involved in an intramolecular disulphide bond. C2 and C6 are involved in the interaction with TGFβ-LAP. (ii) Schematic diagram of a representative cbEGF domain with conserved cysteine residues labelled and disulphide bonds illustrated by lines. The position of the substituted cysteine, C1286, C5 is indicated by an arrow. (C) SDS-PAGE of the purified LTBP4 N- and C-terminal constructs: NWT, C274G, CWT, C1286S and C1186R under reducing (R) and non-reducing (NR) conditions.