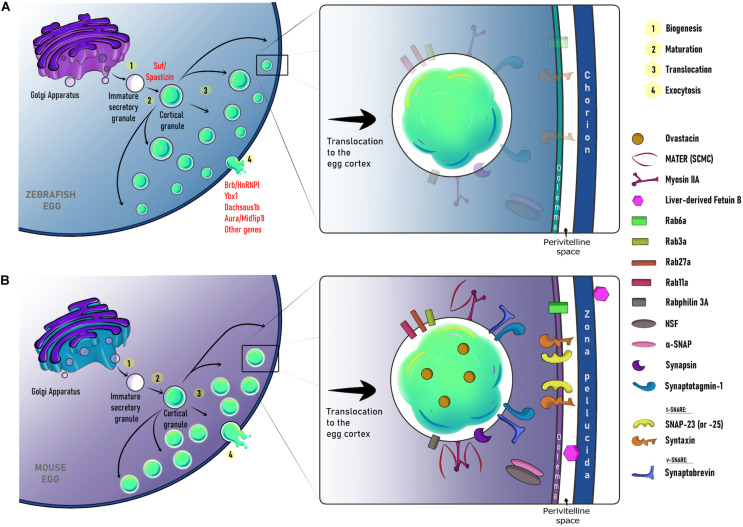
FIGURE 3.
Schematic representation of major steps and molecular regulators for cortical granule biogenesis, translocation, and exocytosis in the zebrafish (A) and mouse (B). In the early oocyte, CGs are formed from the Golgi as immature secretory vesicles. In zebrafish, their maturation is regulated by Suf/Spastizin. During mid and late oogenesis, mature CGs are recruited and translocated to the oocyte cortex. This process is coordinated by an actin-based network and several maternal factors including Rab27a and Rab11a. Ultimately, MATER functions to anchor CGs at the cell cortex. By egg activation/fertilization, their content is exocytosed. This process is regulated by maternally-loaded molecules such as Brb/HnRNP I, Ybx1, Dachsous 1b, Aura/Mid1p1l in the zebrafish egg, and Rab3a, Rabphilin 3A, Rab6a, and the SNARE complex in the mouse egg. Notice that shaded proteins represent those maternally-loaded molecules that could also be present in the zebrafish female gamete. CG, cortical granule; SCMC, subcortical maternal complex.