Figure 1.
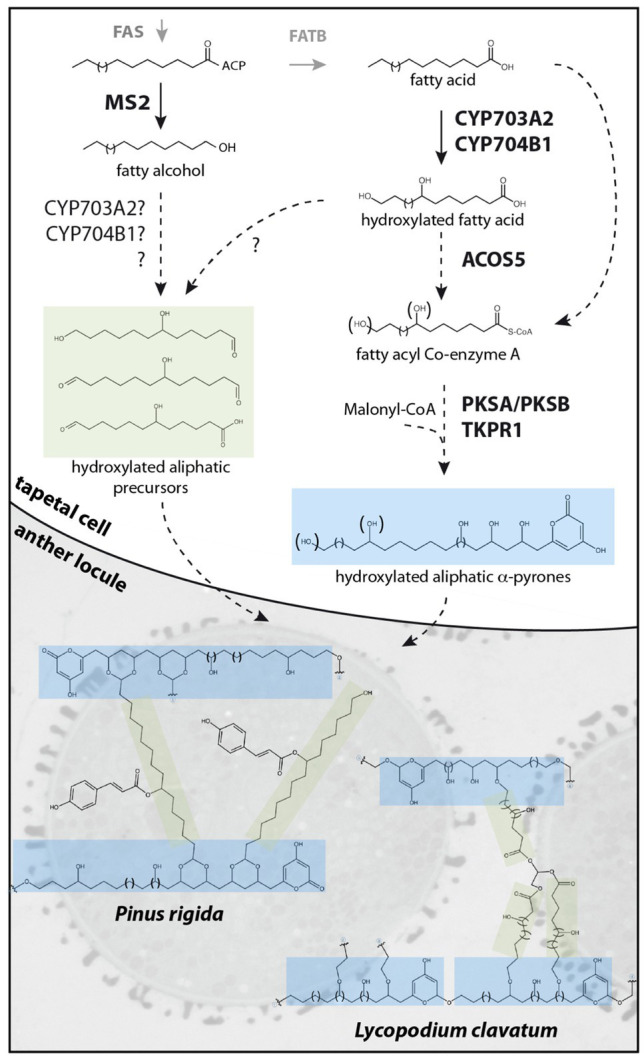
Proposed sporopollenin metabolic pathway and simplified molecular structures of Pinus rigida and Lycopodium clavatum sporopollenin. Fatty acyl-ACP esters, synthesized de novo by the FAS complex, are reduced by MS2 to produce fatty alcohols or hydrolyzed by FATB to produce free fatty acids. After hydroxylation by CYP703A2 and CYP704B1, free fatty acids may be reduced by unknown reductases to fatty alcohols or aldehydes, forming putative hydroxylated aliphatic sporopollenin precursors. Alternatively, hydroxylation by CYP703A2 and CYP704B1 of the fatty alcohol produced by MS2 may occur. In the case of Pinus rigida, these aliphatic precursors might be acylated with phenolics by an unknown transferase. Hydroxylated or non-hydroxylated fatty acids are esterified to CoA by ACOS5 prior to several cycles of condensation by PKSs and reduction by TKPRs to form polyhydroxylated α-pyrone precursors. The precursors could be exported to the anther locule and polymerized on the surface of developing microspores by an unknown mechanism. In Pinus rigida, a simplified sporopollenin is proposed to predominantly contain hydroxylated aliphatic α-pyrone units, crosslinked at one end through an ester group and linked through a dioxane moiety to other units by a hydroxylated aliphatic chain bearing a coumaroyl moiety. In Lycopodium clavatum, the sporopollenin structure is proposed to contain a rigid macrocyclic backbone of several hydroxylated aliphatic α-pyrone units coupled through ether bonds to hydroxylated aliphatic networks linked together by glycerol.
