Abstract
BACKGROUND/OBJECTIVES
Psidium guajava L. (guava) leaves have been shown to exhibit hypoglycemic and antidiabetic effects in rodents. This study investigated the effects of guava leaf extract on adipogenesis, glucose uptake, and lipolysis of adipocytes to examine whether the antidiabetic properties are mediated through direct effects on adipocytes.
MATERIALS/METHODS
3T3-L1 cells were treated with 25, 50, 100 µg/mL of methanol extract from guava leaf extract (GLE) or 0.1% dimethyl sulfoxide as a control. Lipid accumulation was evaluated with Oil Red O Staining and AdipoRed assay. Immunoblotting was performed to measure the expression of adipogenic transcription factors, fatty acid synthase (FAS), and AMP-activated protein kinase (AMPK). Glucose uptake under basal or insulin-stimulated condition was measured using a glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose. Lipolysis from fully differentiated adipocytes was measured by free fatty acids release into the culture medium in the presence or absence of epinephrine.
RESULTS
Oil Red O staining and AdipoRed assay have shown that GLE treatment reduced lipid accumulation during adipocyte differentiation. Mitotic clonal expansion, an early essential event for adipocyte differentiation, was inhibited by GLE treatment. GLE inhibited the expression of transcription factors involved in adipocyte differentiation, such as peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding protein α (C/EBPα), and sterol regulatory element-binding protein-1c (SREBP-1c). FAS expression was also decreased while the phosphorylation of AMPK was increased by GLE treatment. In addition, GLE increased insulin-induced glucose uptake into adipocytes. In lipid-filled mature adipocytes, GLE enhanced epinephrine-induced lipolysis but reduced basal lipolysis dose-dependently.
CONCLUSIONS
The results show that GLE inhibits adipogenesis and improves adipocyte function by reducing basal lipolysis and increasing insulin-stimulated glucose uptake in adipocytes, which can be partly associated with antidiabetic effects of guava leaves.
Keywords: Adipogenesis, lipolysis, Psidium guajava, hypoglycemic agents, 3T3-L1 cells
INTRODUCTION
Diabetes is the most common metabolic disease, accounting for 463 million cases reported from the global population in 2019 [1]. Type 2 diabetes mellitus (T2DM) makes up approximately 90% of diabetes cases. It is caused by relative insulin deficiency and insulin resistance in target organs such as the liver, muscles, and adipose tissues [2]. Since the resulting chronic hyperglycemia leads to the development of diabetes complications [3], the treatment of T2DM requires controlling blood glucose levels and reducing insulin resistance. Several drugs are being used in the clinic to increase insulin sensitivity. However, currently available drugs for T2DM have numerous adverse effects, such as weight gain, congestive heart failure, and increased risk of liver disease [4]. For this reason, the search for antidiabetic agents has been directed toward plants used in traditional medicine.
Psidium guajava L. (guava) has been consumed not only as food but also as traditional medicine for pharmacological purposes, such as antioxidant, antimicrobial, anti-inflammatory, antispasmodic, and antidiabetic effects [5]. In East Asia and South Africa, guava leaves have been widely used for a long time for the treatment of diabetes [5]. The antidiabetic activities of guava leaves have been reported in previous studies. The aqueous and ethanolic extracts of guava leaves decreased blood glucose and HbA1c levels and reduced postprandial glucose elevation in diabetic rodent models [6,7] and human trials with prediabetic and T2DM patients [8]. Although the mechanisms underlying the antidiabetic properties of guava leaves are not yet fully perceived, the inhibition of α-glucosidase by guava leaf extract (GLE) has been suggested to be associated with an attenuated elevation of postprandial blood glucose levels [8,9]. Additionally, GLE could influence the metabolism of insulin-target tissues. It was reported that GLE increased glucose uptake in hepatocytes [10] and inhibited hepatic gluconeogenesis by increasing glycogen synthesis in diabetic rats [11]. Glucose uptake into muscle cells was also stimulated by GLE [12].
Considering that adipose tissue is a major insulin target tissue and impaired glucose uptake in adipose tissue can cause insulin resistance [13], the effect of GLE on adipocytes should be investigated, in addition to hepatocytes and muscle cells. Adipocytes regulate energy balance by storing triacylglycerol (TG) or mobilizing fatty acids in response to changes in energy status. Excessive fat accumulation in adipose tissue occurs in obesity and leads to obesity-induced insulin resistance and T2DM by increasing inflammatory cytokines and free fatty acids release from adipocytes [14,15]. Adipose tissue can be expanded by either the increase in the number or size of adipocytes. Preadipocytes develop into lipid-filled mature adipocytes by the process of adipogenesis [16]. It is closely associated with the etiology of obesity [17]. During adipocyte differentiation, activation of transcription factors, including peroxisome proliferator activated receptor γ (PPARγ), CCAAT/enhancer-binding protein (C/EBP), and sterol regulatory element-binding protein-1c (SREBP-1c) are required to express lipogenic genes, such as acetyl-CoA carboxylase and fatty acid synthase (FAS) [18]. Recently, it has been reported that GLE reduced body weight gain and adipose tissue weight in diabetic rodents [19,20]. Nevertheless, little is known about whether GLE can affect adipogenesis. In this study, the effect of GLE on adipocyte differentiation and adipocyte metabolic function, such as lipolysis and glucose uptake, was investigated to understand the cellular mechanisms underlying the antidiabetic effects of GLE.
MATERIALS AND METHODS
Chemicals and preparation of GLE
Fetal bovine serum (FBS), bovine serum (BS), penicillin-streptomycin, Dulbecco's Modified Eagle medium (DMEM), and 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxy-D-glucose (2-NBDG) were purchased from Invitrogen (Carlsbad, CA, USA). Antibodies specific for PPARγ, SREBP-1c, and FAS were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against C/EBPα, AMP-activated protein kinase (AMPK), and phosphor-Thr 172-AMPK were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies specific for GAPDH and α-tubulin were purchased from Ab Frontier (Seoul, Korea). Other chemicals were purchased from Sigma-Aldrich (St, Louis, MO, USA) when origin was not specified.
Guava leaves were obtained from a farm on Jeju Island (Jeju, Korea). The leaves (100 g) were air-dried, crushed, and extracted with MeOH for 24 h at room temperature. After filtration, the extract was concentrated in a rotary evaporator to obtain 27 g of GLE, which was stored in a freezer until use.
Cell culture and differentiation
3T3-L1 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cell culture was performed as described previously [21,22]. Briefly, cells were grown in 10% BS/DMEM until 2 days after confluence and differentiated with induction medium containing 10% FBS, 0.5 µM isobutylmethylxanthine (IBMX), 1 µM dexamethasone, and 167 nM insulin (D0). After 2 days, cells were cultured with 10% FBS/DMEM and 167 nM insulin for another 2 days and then maintained with 10% FBS/DMEM until reaching lipid-filled phenotype.
Cell viability assay
Cell viability was monitored by MTS assay (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt, MTS; Celltiter 96 Aqueous One solution, Promega, Madison, WI, USA). For preadipocytes, cells seeded in 96-well plates were treated with GLE (5, 25, 50, 100 µg/mL) in differentiation induction medium after 2 days of cell confluency. For mature adipocytes, GLE (5, 25, 50, 100 µg/mL) was added at 9 days after induction of differentiation. Cells were incubated for 24, 48, and 72 h for preadipocytes and 24 h for mature adipocytes. Next, MTS reagent was added and incubated at 37°C for 1 h. The absorbance was measured at 490 nm to determine the formazan concentration, which is proportional to the number of living cells.
Adipogenesis assay
GLE (25, 50, 100 µg/mL) or dimethyl sulfoxide (DMSO; control) was added to differentiation induction medium for 6 days (D0 to D6) according to the differentiation protocol. To visualize lipids, cells were washed with phosphate-buffered saline (PBS), fixed with 10% formalin, and stained with Oil Red O solution and hematoxylin. Intracellular lipid was quantified by AdipoRed Assay Reagent (Lonza, Walkersville, MD, USA).
Western blotting analysis
Cells were washed with PBS and homogenized in cold radio immune precipitation assay (RIPA) buffer. The cell lysate was centrifuged (10,000 g for 10 min at 4°C) and protein concentration of the supernatant was measured with a protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Supernatants with equal amount of protein were separated in sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels and transferred onto nitrocellulose membranes. The membranes were blocked with 5% skim milk in 10 mM Tris-HCl (pH 6.8), 100 mM NaCl, and 0.1% Tween 20 (TBS-T buffer). After incubation overnight with primary antibody at 4°C, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Bands were detected by an ECL system (Ab Frontier), and the protein expression was quantified by ImageJ Program (NIH, Bethesda, MD, USA).
Glucose uptake
Mature adipocytes seeded on 96-well black plates were preincubated for 2 h with serum-free DMEM and then incubated with GLE (25, 50, 100 µg/mL) for 24 h to investigate the effect of GLE on basal glucose uptake. In addition, the effect of GLE on insulin-induced glucose uptake was examined by adding 100 nM insulin at 30 min before the end of treatment. After washing with serum-free DMEM, 20 µM 2-NBDG was added and incubated for 30 min. Cells were washed with PBS to remove free 2-NBDG and the fluorescence was measured with a fluorescence microplate reader, with an excitation wavelength of 465 nm and an emission wavelength of 540 nm.
Lipolysis assay
Fully differentiated adipocytes were incubated in Krebs-Ringer bicarbonate buffer (119 mM NaCl, 4.8 mM KCl, 1.28 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM 7H2O·MgSO4, 0.25 mM NaHCO3, 5 mM glucose, 4% bovine serum albumin, pH 7.4) containing GLE (25, 50, 100 µg/mL) or DMSO in the presence or absence of epinephrine (1 µM). After 6 h, the conditioned medium was collected from each well and nonesterified fatty acid (NEFA) was measured with an assay kit (Wako, Osaka, Japan). The content was calibrated by pellet protein concentration.
Statistical analyses
Statistical analysis was performed by one-way analysis of variance followed by Duncan's multiple range test using SAS software (version 9.4, SAS Institute, Inc., Cary, NC, USA). A P-value < 0.05 was considered significant. Data are expressed as the mean ± SD with indications of the number of replicates.
RESULTS
GLE inhibits lipid accumulation during adipogenesis
All concentrations of GLE (5, 25, 50, 100 µg/mL) did not show cytotoxicity in either preadipocytes or mature adipocytes after 24 h treatment (data not shown). Thus, concentrations of GLE up to 100 µg/mL were treated to the cells in the experiments.
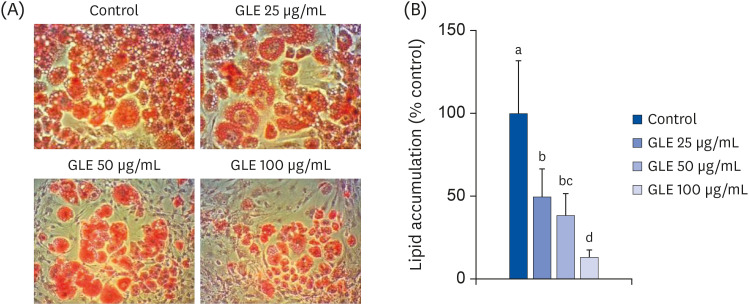
To investigate the effect of GLE on lipid accumulation during differentiation, Oil Red O staining and AdipoRed assay were performed. Oil Red O staining showed that GLE decreased cellular lipid accumulation (Fig. 1A). It was supported by quantitative results from AdipoRed assay (Fig. 1B). GLE decreased the lipid content by 51%, 62%, and 88% at 25, 50, and 100 µg/mL, respectively, compared with the control (P < 0.001).
Fig. 1. Effect of MeOH extract from GLE on differentiation of 3T3-L1 cells. Cells were induced to differentiate with induction media including GLE (25, 50, and 100 µg/mL) or DMSO (control) for 6 days. (A) Oil Red O staining (100×). (B) Intracellular lipid content by AdipoRed assay. Values are the mean ± SD (n = 8).
GLE, guava leaf extract; DMSO, dimethyl sulfoxide.
a-dMeans without the same letter are significantly different, as determined by analysis of variance followed by Duncan's test (P < 0.001).
GLE suppresses the expression of adipogenic transcription factors and fatty acid synthase via AMPK signaling
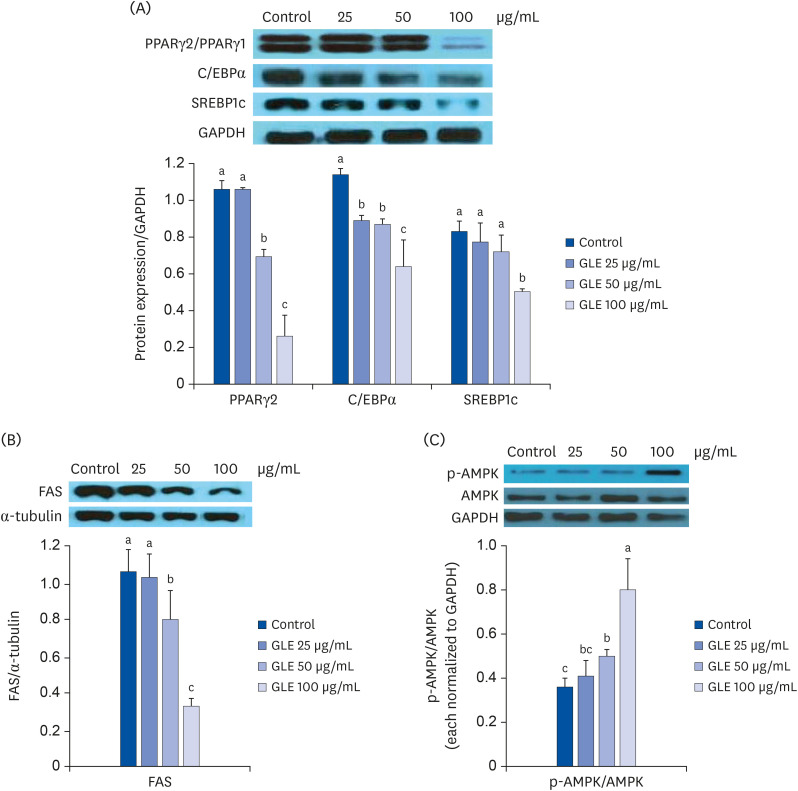
The expression of adipogenic transcription factors in cells treated with GLE was determined at D6 by Western blot analysis. The results are shown in Fig. 2A. The expression of PPARγ, a major master regulator of adipocyte differentiation, was reduced by 35% and 76% with GLE treatment at 50 and 100 µg/mL, respectively, compared with the control (P < 0.001). The expression of C/EBPα and SREBP-1c was also lowered in cells incubated with GLE by 44% and 40% relative to the control at 100 µg/mL, respectively. GLE also decreased the expression of FAS, a late adipogenic marker enzyme, markedly at 50 and 100 µg/mL (P < 0.001; Fig. 2B).
Fig. 2. Effect of MeOH extract from GLE on the expression of adipogenesis-related factors. 3T3-L1 cells were induced to differentiate in the presence of GLE (25, 50, and 100 µg/mL) or DMSO (control). (A) Western blot analysis of PPARγ, C/EBPα, and SREBP1c measured after 6 days of differentiation induction. (B) Western blot analysis of fatty acid synthase measured after 6 days of differentiation induction (FAS). (C) AMPK activation at 24 h after induction of differentiation. The protein levels of p-AMPK were measured and normalized to those of AMPK. Values are the mean ± SD (n = 3).
GLE, guava leaf extract; DMSO, dimethyl sulfoxide; PPARγ, peroxisome proliferator activated receptor γ; C/EBPα, CCAAT/enhancer-binding protein α; SREBP-1c, sterol regulatory element-binding protein-1c; FAS, fatty acid synthase; AMPK, AMP-activated protein kinase.
a-cMeans without the same letter are significantly different, as determined by analysis of variance followed by Duncan's test (P < 0.05).
To investigate the signaling pathway responsible for the inhibition of adipogenesis by GLE, the effect of GLE on AMPK phosphorylation was examined during the early phase of adipogenesis. At 24 h after induction of differentiation, the phosphorylation of AMPK was increased in cells treated with GLE at concentrations of 50 and 100 µg/mL, approximately up to 2.3-fold relative to the control cells (Fig. 2C). This result suggests that GLE inhibits adipogenesis by activating the AMPK pathway in 3T3-L1 cells.
GLE inhibits mitotic clonal expansion
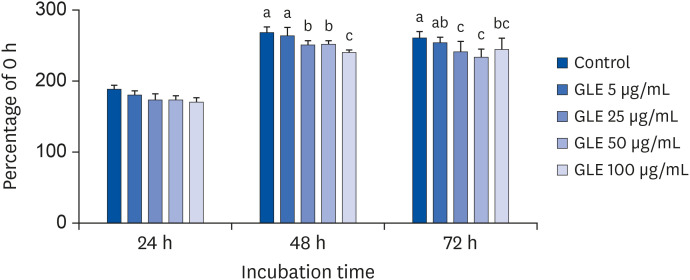
Induction of differentiation causes mitotic clonal expansion, in which growth-arrested postconfluent preadipocytes reenter cell replication and subsequent irreversible commitment to differentiation [23]. To observe whether GLE affects the initiation phase of adipogenesis, we monitored the number of viable cells over 3 days after inducing differentiation. As shown in Fig. 3, GLE (25, 50, 100 µg/mL) significantly reduced the number of cells after 48 and 72 h treatment compared with control (P < 0.001). This result indicates that GLE inhibits adipogenesis by suppressing cell proliferation during mitotic clonal expansion in 3T3-L1 cells.
Fig. 3. Effect of MeOH extract from GLE on cell viability of 3T3-L1 cells during postconfluent mitotic clonal expansion. Cells were treated with GLE (5, 25, 50, and 100 µg/mL) or DMSO (control) with MDI differentiation medium at D0. After 24, 48, and 72 h of treatment, viability was measured by MTS assay.
GLE, guava leaf extract; DMSO, dimethyl sulfoxide; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
a-cMeans without the same letter are significantly different, as determined by analysis of variance followed by Duncan's test (P < 0.001).
GLE increases insulin-stimulated glucose uptake
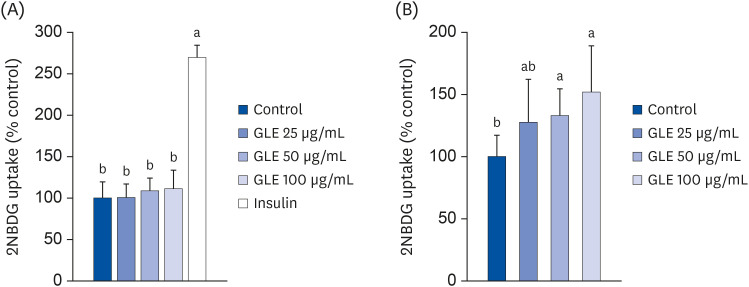
To investigate the effect of GLE on glucose uptake by adipocytes, 3T3-L1 cells were treated with GLE and glucose uptake was measured in the basal and insulin-stimulated conditions. As shown in Fig. 4A, GLE did not affect basal glucose uptake, while insulin increased glucose uptake approximately 2.7-fold in adipocytes. However, GLE treatment in combination with insulin significantly increased 2-NBDG uptake (P < 0.05; Fig. 4B). At a concentration of 100 µg/mL, the increase in 2-NBDG uptake was up to 52%, compared to the incubation with 100 nM insulin alone.
Fig. 4. Effect of MeOH extract from GLE on glucose uptake in 3T3-L1 cells. Cells were treated with GLE (25, 50, and 100 µg/mL) or DMSO (control) for 24 h in basal condition (A) or 100 nM insulin-stimulated condition (B). Insulin was added at 30 min before the end of incubation and then 20 µM 2-NBDG (a fluorescent glucose analog) was treated for 30 min. Values are the mean ± SD (n = 8).
GLE, guava leaf extract; DMSO, dimethyl sulfoxide; 2-NBDG, 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxy-D-glucose.
a,bMeans without the same letter are significantly different, as determined by analysis of variance followed by Duncan's test (P < 0.05).
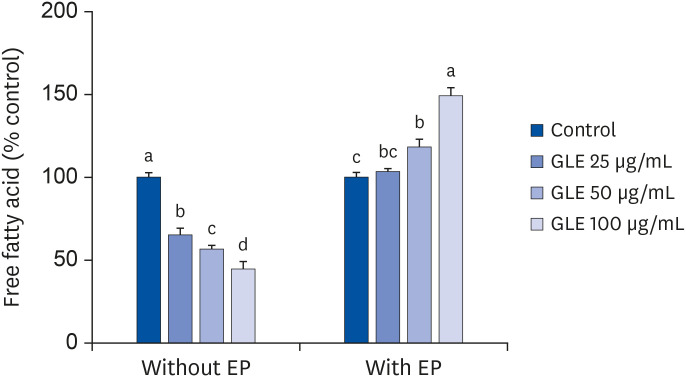
Effect of GLE on adipocytes lipolysis
The unstimulated basal lipolysis in fully differentiated adipocytes was reduced by the treatment of GLE dose dependently (P < 0.001; Fig. 5). After 6 h of treatment with 100 µg/mL GLE, NEFA released into the culture medium was reduced by 55% compared with the control. On the contrary, epinephrine-induced lipolysis was increased by GLE treatment. Adipocytes incubated with 100 µg/mL GLE showed an approximately 50% increase in NEFA release relative to the control.
Fig. 5. Effect of MeOH extract from GLE on lipolysis in mature 3T3-L1 adipocytes. Fully differentiated adipocytes were treated with GLE (25, 50, and 100 µg/mL) or DMSO (control) for 6 h with or without 1 μM EP. Values are the mean ± SD (n = 6).
GLE, guava leaf extract; DMSO, dimethyl sulfoxide; EP, epinephrine.
a-dMeans without the same letter are significantly different, as determined by analysis of variance followed by Duncan's test (P < 0.05).
DISCUSSION
In the present study, we investigated the effect of guava leaves on adipocytes with regard to their antidiabetic properties. The results demonstrate that GLE has multiple effects on adipocytes, including adipogenesis, glucose uptake, and lipolysis.
Obesity is characterized by increased adipose tissue mass, which is linked to the onset of insulin resistance and T2DM. Several studies have reported that oral administration of GLE reduced body weight and adipose tissue weight [19,20]. Adipose tissue mass can be expanded by adipocyte differentiation and subsequent fat accumulation [16]. In this study, GLE treatment during differentiation inhibited lipid accumulation, as shown by Oil Red O staining and a dose-dependent decrease in lipid content. In addition, GLE significantly downregulated the expression of transcription factors associated with adipogenesis, such as PPARγ, C/EBPα, and SREBP-1c, compared to control adipocytes. These transcription factors are expressed during adipocyte differentiation, before the activation of adipocyte-specific gene expression [24]. PPARγ, C/EBPα, and SREBP-1c activate the downstream promoters of adipocyte-specific genes such as FAS, lipoprotein lipase (LPL), fatty acid binding protein (FABP), acyl-CoA synthase (ACS) 1, and leptin [18]. Among the target proteins, we measured the expression of FAS, a terminal marker of adipocyte differentiation protein. FAS plays a crucial role in fatty acid synthesis and lipogenesis. GLE treatment decreased the expression of FAS, as expected. Therefore, reducing fat accumulation through the inhibition of adipocyte differentiation may be an underlying mechanism of anti-diabetic effects.
Adipocyte differentiation occurs by two consecutive essential events: mitotic clonal expansion and irreversible commitment to differentiation [23]. During mitotic clonal expansion after initiation of differentiation, cells are restored from growth arrest induced by cell confluence and undergo several rounds of replication. In this study, we found that GLE had an inhibitory effect on mitotic clonal expansion. Considering that adipose tissue mass is determined by the number and size of adipocytes, the fewer adipocytes by inhibition of mitotic clonal expansion could be related to the GLE-mediated decrease in lipid accumulation. Various phytochemicals from natural sources had inhibitory effects on adipocyte lipid accumulation through suppression of mitotic clonal expansion [25]. It has been suggested that mitotic clonal expansion could be an important target in developing anti-obesity products.
AMPK has been shown to play a crucial role in regulating lipid and glucose metabolism in various tissues, such as the liver, adipose tissue, and muscles [26,27]. Because AMPK stimulates the expression or phosphorylation of downstream target genes involved in energy metabolism when activated, the AMPK cascade could be a target for the treatment of obesity and related metabolic diseases. In this study, treatment with GLE induced the activation of AMPK. Several studies have shown that AMPK activation can inhibit adipocytes differentiation by downregulating the expression of adipogenic transcription factors and their target enzymes [28,29]. Furthermore, it was reported that the treatment of 3T3-L1 cells with AMPK activator in the early stage of differentiation inhibited mitotic clonal expansion dose-dependently [29,30], which is consistent with our results. Therefore, it is suggested that GLE effectively inhibits lipid accumulation by attenuating the expression of key adipogenic transcription factors through activation of AMPK during the early phase of adipocyte differentiation.
In response to energy demand, TG stores in adipocytes are hydrolyzed by lipases, which are regulated by hormone and biochemical signals. The released free fatty acids are transported to other tissues for use. There are few studies investigating the effect of GLE on lipolysis in adipocytes. In this study, GLE treatment enhanced free fatty acid release from lipid-filled mature adipocytes when lipolysis was stimulated by epinephrine. Considering that catecholamine-induced lipolysis was impaired in obesity [31], lipid mobilization by increasing epinephrine-induced lipolysis could be another way of reducing adipose tissue mass.
On the other hand, GLE treatment strongly inhibited basal lipolysis of mature 3T3-L1 cells in this study. Unstimulated basal lipolysis is increased in expanded mature adipocytes, which is closely associated with insulin resistance [32]. Elevated circulating fatty acids have been suggested to modify adipokine secretion and induce ectopic fat deposition in skeletal muscles and the liver [33]. These alterations lead to decreased glucose utilization in muscles and stimulate glucose production in the liver [34,35]. Reducing basal lipolysis is important to increase insulin sensitivity. Therefore, the basal antilipolytic effect of GLE in this study could contribute to the antidiabetic effect of guava leaves observed in previous studies.
Glucose uptake into peripheral tissues is an effective way to control blood glucose levels. GLE did not affect basal glucose uptake into adipocytes in this study, while GLE has been reported to increase basal glucose uptake in hepatocytes and C2C12 muscle cells [10,12]. In contrast to low glucose uptake under basal conditions, insulin remarkably stimulates glucose uptake into the target tissues through the insulin signaling pathway. It has been reported that GLE treatment promoted the translocation of glucose transporter type 4 and activation of AMPK and proteins associated with the insulin signaling pathway, such as IRS-1, PI3K, and Akt, in skeletal muscle of diabetic rats, although no data were provided regarding glucose uptake into the cells [19]. This study shows that GLE treatment increased insulin-stimulated 2-NBDG uptake in 3T3-L1 cells. This finding indicated that GLE could exert a hypoglycemic effect by promoting insulin-stimulated glucose uptake into adipocytes as well as skeletal muscle. This study was limited in that the mechanism underlying glucose uptake increase in adipocytes was not investigated. While AMPK is known to increase glucose uptake through increased GLUT-4 translocation in skeletal muscle [36], the effect of AMPK activation is not consistent in adipose tissue [37]. Further research is needed to investigate the effect of GLE treatment on the insulin signaling pathway and AMPK with regard to glucose transport into adipose tissue. Nevertheless, to our knowledge, this is the first study to show the direct actions of GLE on adipocytes.
In conclusion, our study has shown that GLE suppresses lipid accumulation during adipocyte differentiation and improves adipocyte metabolic function by reducing basal lipolysis and enhancing insulin-stimulated glucose uptake. The results support the effectiveness of GLE as a therapeutic agent for treating diabetes.
Footnotes
Funding: This study was supported by the Catholic University of Korea, Research Fund, 2020.
Conflict of Interest: The authors declare no potential conflicts of interests.
- Conceptualization: Kim HK, Choi E.
- Formal analysis: Choi E, Baek S.
- Funding acquisition: Kim HK.
- Investigation: Choi E, Baek K.
- Methodology: Kim HK, Choi E.
- Writing - original draft: Choi E.
- Writing - review & editing: Kim HK, Baek K, Baek S.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 9th Edition. Brussels, Belgium: International Diabetes Federation; 2019. [cited 2020 August 10]. Available from: https://www.diabetesatlas.org/ [Google Scholar]
- 2.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 3.Giaccari A, Sorice G, Muscogiuri G. Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutr Metab Cardiovasc Dis. 2009;19:365–377. doi: 10.1016/j.numecd.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Kalsi A, Singh S, Taneja N, Kukal S, Mani S. Current treatments for type 2 diabetes, their side effects and possible complementary treatments. Int J Pharm Pharm Sci. 2015;7:13–18. [Google Scholar]
- 5.Gutiérrez RM, Mitchell S, Solis RV. Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2008;117:1–27. doi: 10.1016/j.jep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Khan HB, Shanmugavalli R, Rajendran D, Bai MR, Sorimuthu S. Protective effect of Psidium guajava leaf extract on altered carbohydrate metabolism in streptozotocin-induced diabetic rats. J Diet Suppl. 2013;10:335–344. doi: 10.3109/19390211.2013.830677. [DOI] [PubMed] [Google Scholar]
- 7.Ojewole JA. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27:689–695. doi: 10.1358/mf.2005.27.10.948917. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi Y, Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond) 2010;7:9. doi: 10.1186/1743-7075-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Liu HC, Hong JR, Li HG, Huang CY. Effect of Psidium guajava leaf extract on alpha-glucosidase activity in small intestine of diabetic mouse. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:298–301. [PubMed] [Google Scholar]
- 10.Shen SC, Cheng FC, Wu NJ. Effect of guava (Psidium guajava Linn.) leaf soluble solids on glucose metabolism in type 2 diabetic rats. Phytother Res. 2008;22:1458–1464. doi: 10.1002/ptr.2476. [DOI] [PubMed] [Google Scholar]
- 11.Vinayagam R, Jayachandran M, Chung SS, Xu B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018;103:1012–1017. doi: 10.1016/j.biopha.2018.04.127. [DOI] [PubMed] [Google Scholar]
- 12.Beidokhti MN, Eid HM, Villavicencio MLS, Jäger AK, Lobbens ES, Rasoanaivo PR, McNair LM, Haddad PS, Staerk D. Evaluation of the antidiabetic potential of Psidium guajava L. (Myrtaceae) using assays for α-glucosidase, α-amylase, muscle glucose uptake, liver glucose production, and triglyceride accumulation in adipocytes. J Ethnopharmacol. 2020;257:112877. doi: 10.1016/j.jep.2020.112877. [DOI] [PubMed] [Google Scholar]
- 13.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 14.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 15.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 16.Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013;92:229–236. doi: 10.1016/j.ejcb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 18.Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 2016;17:124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Yoshitomi H, Gao M, Qin L, Duan Y, Sun W, Xu T, Xie P, Zhou J, Huang L, Liu T. Guava leaf extracts promote glucose metabolism in SHRSP.Z-Leprfa/Izm rats by improving insulin resistance in skeletal muscle. BMC Complement Altern Med. 2013;13:52. doi: 10.1186/1472-6882-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Wen YM, Shen J, Chen MM, Wen JH, Li ZM, Liang YZ, Xia N. Guava leaf extract attenuates insulin resistance via the PI3K/Akt signaling pathway in a type 2 diabetic mouse model. Diabetes Metab Syndr Obes. 2020;13:713–718. doi: 10.2147/DMSO.S231979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim SY, Shin YE, Kim HK. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr Res. 2019;65:54–62. doi: 10.1016/j.nutres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim JN, Han SN, Kim HK. Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3T3-L1 cells. Nutr Res. 2014;34:723–731. doi: 10.1016/j.nutres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E, Kim CY. Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. 2019;24:1157. doi: 10.3390/molecules24061157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. 2013;366:194–203. doi: 10.1016/j.mce.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Kola B, Grossman AB, Korbonits M. The role of AMP-activated protein kinase in obesity. Front Horm Res. 2008;36:198–211. doi: 10.1159/000115366. [DOI] [PubMed] [Google Scholar]
- 28.Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Wang D, Zhu Q, Gao X, Yang S, Xu A, Wu D. Inhibitory effects of A-769662, a novel activator of AMP-activated protein kinase, on 3T3-L1 adipogenesis. Biol Pharm Bull. 2009;32:993–998. doi: 10.1248/bpb.32.993. [DOI] [PubMed] [Google Scholar]
- 30.Habinowski SA, Witters LA. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2001;286:852–856. doi: 10.1006/bbrc.2001.5484. [DOI] [PubMed] [Google Scholar]
- 31.Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Rydén M, Arner E, Sicard A, Jenkins CM, Viguerie N, van Harmelen V, Gross RW, Holm C, Arner P. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54:3190–3197. doi: 10.2337/diabetes.54.11.3190. [DOI] [PubMed] [Google Scholar]
- 32.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003;284:E863–73. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- 36.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond) 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]