Abstract
Background
Nedocromil sodium and sodium cromoglycate inhaled shortly before exercise appear to reduce the severity of exercise‐induced bronchoconstriction. There is some debate over which drug may be more effective.
Objectives
The objective of this review was to compare the effects on post‐exercise lung function between prophylactic doses of nedocromil sodium (NSG) and sodium cromoglycate (SCG) in persons diagnosed with exercise‐induced bronchoconstriction.
Search methods
Randomized controlled trials were identified from the Cochrane Airways Review Group Asthma Register. Bibliographies of relevant studies and review articles were searched and primary authors, content experts and manufacturers of drugs were contacted for additional relevant studies. No language restrictions were applied. Searches were current as of December 2007.
Selection criteria
Randomized controlled trials comparing NCS to SCG in prophylactic treatment of exercise‐induced bronchoconstriction were eligible. Studies were included if: the participants, aged 6 or over, had a confirmed diagnosis of asthma with exercise‐induced bronchoconstriction, were subjected to an exercise challenge sufficient to trigger bronchoconstriction, and the measures of lung function were reported as either changes in forced expiratory volume in one second or peak expiratory flow rate.
Data collection and analysis
Data extraction and methodological quality assessments were conducted independently by two reviewers using standard forms and validated assessment criteria. In some cases results were extrapolated from graphs. Results from similar studies were pooled and reported as the weighted mean difference (WMD) or odds ratio (OR) with 95% confidence intervals (CI) using the random effects model.
Main results
Nine studies (162 participants) are included in this review. No significant difference was noted between NCS and SCG with respect to the maximum percent decrease in FEV1 (WMD = ‐0.88; 95% CI: ‐4.50, 2.74), complete protection (i.e. maximum % fall FEV1 still =>10%); OR = 0.95; 95% CI: 0.50 to 1.8, clinical protection (i.e. < 50% improvement over placebo); OR = 0.71; 95% CI: 0.36 to 1.39; unpleasant taste (OR = 6.85; 95% CI: 0.77, 60.73), or sore throat (OR = 3.46; 95% CI: 0.32, 37.48). For these pooled comparisons, no statistically significant heterogeneity was identified. Subgroup analyses based on age, dosage of medications and timing of exercise post‐inhalation were consistent with the overall pooled analyses.
Authors' conclusions
No significant differences were evident between the effect of NCS and SCG during the immediate post‐exercise period in adults and children with EIB with regards to pulmonary function ‐ specifically maximum percent decrease in FEV1, complete protection, clinical protection, or side effects.
Plain language summary
Nedocromil sodium versus sodium cromoglycate for preventing exercise‐induced bronchoconstriction
Exercise‐induced asthma can limit people's endurance, prolong recovery time after exercise, and lead to people avoiding exercise. The episode involves symptoms such as coughing, wheezing, shortness of breath and chest tightness. Two drugs, nedocromil sodium (Tilade) and sodium cromoglycate are sometimes used before exercise to prevent asthma. The review of trials found that both were similarly effective in relieving exercise‐induced asthma for both children and adults. More people have sore throats and an unpleasant after‐taste after using nedocromil sodium.
Background
Airway hyper‐reactivity that leads to airway narrowing following an exercise challenge is a phenomenon known as exercise induced bronchoconstriction (EIB) or exercise‐induced asthma (EIA). It occurs in 70% ‐ 80% of people with asthma (Anderson 1985) and an estimated 12% ‐15% of the general population (Spector 1993).
EIB is characterized by a transient decrease in the forced expiratory volume in 1 second (FEV1) or in the peak expiratory flow rate (PEFR) provoked by 6 to 15 minutes of continuous, strenuous exercise (Bar‐Yishay 1984). By consensus, post‐exercise decreases of 10% to 20% in FEV1 or PEFR indicate mild EIB; falls of 20% ‐ 40% represent moderate EIB, and > 40% represents severe EIB. (Eggleston 1984). The airflow obstruction causes dyspnea, cough, wheeze, premature fatigue, and prolonged recovery times. Maximum broncho‐constriction typically occurs 5 to 15 minutes following exercise and usually subsides spontaneously within 20 to 60 minutes (Virant 1992). The severity and impact of symptoms is dependent on several factors: concomitant asthma therapy, intensity and duration of activity, environmental conditions, degree of underlying bronchial hyper‐reactivity, level of physical conditioning, and the time interval since previous exercise (Rupp 1996).
Management of EIB has been the focus of intense pharmacotherapeutic research and the emphasis has been on prophylactic therapy. Different drugs have proven useful; however, there remains considerable debate regarding the merits, the optimal dose and the best delivery method for each treatment. Traditionally, inhaled beta‐agonists and other bronchodilating agents have been the drugs of choice (Virant 1992; Sly 1984). Recently, the anti‐inflammatory agents nedocromil sodium (NCS), sodium cromoglycate (SCG), and inhaled corticosteroids (ICS) have been evaluated (Spooner 2002). SCG inhibits mast cell degranulation, and can prevent or attenuate bronchospasm induced by exercise or cold air, particularly in children. NCS is a chemically unrelated drug with similar effects (Anon 1999). Nedocromil has been reported to be effective against bronchospasm induced by antigens, fog, cold air, sulfur dioxide and exercise (Holgate 1986). Individual randomized, controlled trials (RCTs) have been conducted to compare NCS with SCG, but to date no systematic overview that combines all trials to obtain a pooled estimate of the difference in the magnitude of effect between these drugs has been published.
This systematic review examines the available evidence from RCTs comparing the prophylactic efficacy of NCS and SCG in preventing or attenuating EIB.
Objectives
The objective of this review was to quantitatively compare the effects of NCS and SCG administered by a pressurized aerosol or metered dose inhaler (MDI) prior to a strenuous exercise challenge in subjects who suffer from EIB.
Methods
Criteria for considering studies for this review
Types of studies
To be considered for inclusion, clinical studies had to be randomized, placebo‐controlled, double blind trials.
Types of participants
Prior to entry into the trial, participants had to have a diagnosis of EIB. This meant they had to demonstrate a reproducible fall in FEV1 of >10% post exercise on a control challenge with no pretreatment. FEV1 or PEFR post‐exercise criteria were used to define EIB severity: 10% to 20% ‐ mild, 20% to 40% ‐ moderate, and > 40% ‐ severe (Anderson 1985; Virant 1992; Eggleston 1984). Studies which did not state specific criteria for EIB or that included people with impairment < 10% were excluded. Studies recruiting children (6 to 17 years.), and adults (=> 18 years.) were included and these age designations formed subgroup analyses.
Types of interventions
Studies reporting results from participants who were randomized to receive a single prophylactic dose of either inhaled NCS or SCG prior to a standardized exercise challenge were included. Studies that had additional drug arms were considered, but only results pertaining to NCS and SCG are included in the present review. Studies that involved delivery using nasal sprays were not included.
Types of outcome measures
Outcomes: All patient outcomes, both subjective and objective, were considered. The primary outcomes of interest were continuous data from pulmonary function measures (FEV1 and PEFR). The conventional method to quantify EIB is to measure the maximum reduction in FEV1 or PEFR and to express it as a percent (%) fall index, that is, to express the reduction in lung function after exercise as a percent of the pre‐exercise value, b) the number of patients who received clinical protection from EIB, c) the number and nature of adverse effects experienced.
Complete protection was not obtained if the percentage drop in FEV1 post exercise was greater than the normal range (>10%), some trialists use > 15%, the diagnostic cut‐point. Clinical protection was not obtained if the percentage fall after receiving the active drug was < 50% of the drop after receiving the placebo (de Benedictis 1994a).
Subgroup analyses was conducted for children and adults; exercise timing, and dosages of NCS and SCG.
Search methods for identification of studies
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms: :
(bronchoconstrict* or bronchospas* or exercis* or physical or train* or EIB or EIA) AND (nedocromil* OR "Nedocromil Sodium*" OR Tilade OR NS*) AND ("Cromolyn Sodium*" OR "Sodium Cromoglycate*" OR SCG* OR CS* OR CR*)
Reference lists of each primary study and review article were checked to identify additional potentially relevant citations. Inquires regarding other published or unpublished studies known and/or supported by the authors of the primary studies were made and results included in this review. Personal contact with colleagues, collaborators and other investigators working in the field of asthma was made to identify potentially relevant studies and the drug company that manufacturers NSC and SCG (Rhone‐Poulenc Rorer) was contacted.
We did not exclude trials on the basis of language. When necessary, attempts were made to translate the articles from the foreign language literature.
The most recent literature search was conducted in December 2007.
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, two reviewers (CS, BR) independently reviewed literature searches to identify potentially relevant trials for full review. From the full text, using specific criteria, two reviewers (KK, BR) independently selected trials for inclusion in this review. Agreement was measured using kappa statistics. Disagreement was resolved by consensus or third party adjudication (CS).
Data extraction and management
Two reviewers (KK, CS) extracted data on study characteristics and outcome measures of efficiency and safety. Primary study authors were requested to confirm data extraction and provide additional clarification and information. Most authors could not access their original data to perform supplemental analyses. In some cases, expansion of graphic representations of data from the manuscripts were used to obtain estimates of outcomes.
Assessment of risk of bias in included studies
Assessment of methodological quality: two reviewers working independently performed quality assessment. Two methods of assessment were used. First, using the Cochrane approach to assessment of allocation concealment, all trials were scored using the following principles: Grade A: Adequate concealment Grade B: Uncertain Grade C: Clearly inadequate concealment
And second, each study was assessed using a 0‐5 scale (Jadad 1996), summarized as follows: 1) Was the study described as randomized (1=yes; 0=no) 2) Was the study described as double‐blind (1=yes; 0=no) 3) Was there a description of withdrawals and dropouts (1=yes; 0=no) 4) Was the method of randomization well described and appropriate (1=yes; 0=no) 5) Was the method of double blinding well described and appropriate (1=yes; 0=no)
Data synthesis
Data for NCS were entered as the experimental group and SCG was entered as the control group. In the graphs, the results that favour NCS are on the left and those for SCG are on the right. Subgroup comparisons are identified in the Comparisons section. For dichotomous variables, individual and pooled statistics were calculated as odds ratios (OR) with 95% confidence intervals (95% CIs); a random effects model was used. For continuous outcomes, individual and pooled statistics were calculated as weighted mean differences (WMD) and 95% CIs using a random effects model.
Two specific subgroup analyses were planned a priori. One was to compare adults (>18 years) with children (< 18 years). The other was to compare the various doses of NCS and SCG. Other sensitivity analyses were conducted on fixed versus random effects and methodological quality (high versus low).
Results
Description of studies
Results of the search
A search of the Cochrane Airways Group trials register yielded 106 references of which 92 (87%) were original publications. Search of the Cochrane Controlled Trials Register (CCTR) resulted in five additional references. An update search conducted in December 2007 identified an additional included study (Cekic 2002).
Independent review of the abstracts and titles of these publications identified 14 potentially relevant studies. The simple agreement for relevance was 99% with a kappa of 0.90. Three potentially relevant citations were added from bibliographic searching of relevant articles and overviews. Independent review of these 17 potentially relevant articles determined that eight trials met the inclusion criteria for this meta‐analysis. One further study was included in the 2007 update of the review. The poor reporting of data from the conference abstract did not enable us to extract and use numerical outcome data.
Included studies
Nine studies are included in the review. With the exception of one study published in 1987, the studies were published after 1990. Five were from centres in Italy, and one each from Australia, UK, the former Yugolslavia and USA.
Method: All studies incorporated a randomized, placebo‐controlled, crossover study design. One study was single‐blind (Cekic 2002), and the remainder were double‐blind trials. A pharmacologic "carryover" effect was unlikely, since both NCS and SCG are topical, short‐acting agents that do not accumulate and both medications are rapidly cleared from the body (CPS 1999). In all studies, the challenges were conducted with a minimum of a 24 hour elapsed time, to ensure drug clearance.
Participants: There were five studies conducted on children (Comis 1993; de Benedictis 1994a; de Benedictis 1994b; de Benedictis 1995; Novembre 1994) three studies involving adults (Cekic 2002; Konig 1987; Sinclair 1990) and one study that combined the age groups (Morton 1992). The majority of studies recruited stable asthmatics with a post exercise fall in FEV1 of at least 15%. One study recruited EIB patients with a fall in FEV1 of at least 20% (Konig 1987). Cekic 2002 was a conference abstract and provided scant details of the eligibility criteria.
Interventions: Trials evaluated a range of doses of both NCS and SCG. The doses for NCS ranged from 4 mg (Comis 1993; de Benedictis 1994a; de Benedictis 1994b; de Benedictis 1995; Konig 1987, Novembre 1994; Sinclair 1990) and 8 mg (Morton 1992). The doses for SCG were 4 mg (Morton 1992), 10 mg (Comis 1993, de Benedictis 1994a, de Benedictis 1994b, de Benedictis 1995, Novembre 1994, Sinclair 1990) and 20 mg (Konig 1987). Cekic 2002 did not provide details of the dose of the drugs assessed. Timings of administration were 15 minutes (Morton 1992), 20 minutes (de Benedictis 1994a; de Benedictis 1994b; de Benedictis 1995; Konig 1987; Novembre 1994) 30 minutes (Comis 1993; Sinclair 1990), 120 minutes (Konig 1987), 140 minutes (de Benedictis 1995), and 240 minutes (Konig 1987) before a standardized exercise challenge of sufficient intensity and duration to induce EIB. All studies conducted the exercise challenge on different days, at the same time of day, and all challenges were conducted indoors, in controlled environments.
Outcomes: The most common outcomes reported were pulmonary functions. Maximum percent fall in FEV1 ((pre‐exercise baseline value ‐ lowest post‐exercise value / pre‐exercise baseline value) x 100%) was the most common outcome reported. If the maximum % fall in FEV1 after treatment was <15% (Morton 1992; Novembre 1994) or > 10% (de Benedictis 1994a; de Benedictis 1994b; de Benedictis 1995; Konig 1987) complete protection was inferred. If percentage fall after receiving the active drug was less than half the percentage drop after receiving placebo, clinical protection was achieved. Side effects were reported ( Morton 1992; Novembre 1994). Other outcomes included: percent fall in FEV1 at various time intervals, maximum percent decrease in FEF25‐75, percent decrease in FEV1 multiplied by minutes (area under the curve), and maximum percent decrease in PEFR. However, the number of studies reporting each of these measures was insufficient for pooling and these outcomes were not included in this review. One study (Sinclair 1990), which analyzed only the change in mean percent FEV1 at different time points, was not included in the meta‐analysis, but the findings are reported separately.
Risk of bias in included studies
Overall, the methodological quality of the included trials was rated as "moderate". All studies were randomized and double blind. Only one study gave a description of withdrawals (Morton 1992), otherwise there were no dropouts. Only one study described the method of randomization or the method of double blinding (Sinclair 1990). Using Jadad's 5 point validity scale, one study scored 5 (Sinclair 1990), one scored 4 (Morton 1992) and the rest scored 3.
Using the Cochrane criteria to assess allocation concealment, one study was rated as having adequate concealment (Sinclair 1990), and the remaining were rated as "uncertain".
Effects of interventions
The results are reported by outcome. The main results were reported as the weighted mean difference (WMD) in the maximum percent fall in FEV1 between inhaled NCS and SCG. Subgroup analyses are reported on adult versus children, doses of NCS and SCG, and timing of exercise post inhalation.
Pulmonary Function Results: A wide variety of pulmonary function measures were reported in the included trials, but the most common measure was maximum percent fall in FEV1 post exercise. Seven trials reported maximum percent fall in FEV1 as an outcome measure. No significant difference between NCS and SCG with respect to the maximum percent decrease in FEV1 was noted (WMD = ‐0.88; CI: ‐4.50 to 2.74), when all studies were pooled. No statistically significant heterogeneity was identified (X2 = 3.80; df = 6; p > 0.10).
Complete Protection: Seven trials reported complete protection as an outcome measure. No significant difference between NCS and SCG with respect to complete protection existed (OR = 0.74; CI: 0.40 to 1.37) during the study period. With the pooling of all studies, no statistically significant heterogeneity was noted (X2 = 6.80; df = 6; p > 0.10).
Clinical Protection: Five trials (n=4 children, n=1 adult) reported clinical protection as an outcome measure. There was no significant difference between NCS and SCG with respect to clinical protection (OR = 0.80; CI: 0.38,1.72). When these studies were pooled, no statistically significant heterogeneity was demonstrated (X2 = .59; df = 4; p > .10). Cekic 2002 reported that 39/45 in nedocromil sodium treated participants and 34/45 participants in the sodium cromoglycate treated group obtaining protection. No details on threshold FEV1 fall criteria were available.
Side Effects: Two trials reported side effects as an outcome measure. NCS was reported to cause unpleasant taste significantly more often (OR = 6.85; CI: 20.77, 17.05). In the one study that reported sore throat, there was no significant difference between NCS and SCG (OR = 3.03; CI: 0.38, 23.79).
Subgroup/Sensitivity Analyses: Subgroup analyses were conducted by age, group with children and adults analyzed separately. The results were similar to the pooled analyses for maximum percent decrease in FEV1, complete protection and clinical protection.
Sensitivity analysis was performed using various dosages of both NCS and SCG, and timing of exercising post inhalation. The results were consistent with the pooled analyses. No statistically significant effect of drug dosages was identified (NCS 4 mg or 8 mg, and SCG 4 mg, 10 mg or 20 mg), in terms of maximum percent decrease in FEV1, complete protection and clinical protection Timing of exercise post inhalation (30 minutes, 31 ‐ 140 minutes, or > 140 minutes) did not affect the difference between the two medications with regard to maximum percent decrease in FEV1, complete protection or clinical protection.
The results are presented using random effects modelling, however analyses using fixed effects modelling yielded similar results. Fixed effects models generated narrower confidence intervals. Since all studies rated moderate quality (Jadad score = 3 ‐ 4) sensitivity analysis based on quality rating was not conducted.
Discussion
This systematic review compared the effects of NCS and SCG administered by a pressurized aerosol prior to a strenuous exercise challenge in participants with EIB. The pooled result failed to demonstrate any significant difference between the effect of these two medications on pulmonary function ‐ specifically maximum percent decrease in FEV1, the drugs' ability to provide complete protection or clinical protection, or side effects. No significant heterogeneity was evident among the pooled trials. Similarly, subgroup analyses based on age and sensitivity analyses taking into consideration various dosages of the two medications and timing of exercise post inhalation, consistently found a non‐significant difference between these two medications.
One study (Sinclair 1990) that was not included in the pooled result because it reported only the percent fall in FEV1 from baseline as an outcome measure, had similar findings. The results of this study indicated that 4 mg of NCS was equivalent to 10 mg of SCG in preventing exercise induced asthma.
Results of recent meta‐analyses showed NCS to be better than placebo in EIB treatment (Spooner 2002). This review failed to demonstrate any differences between NCS and SCG, however additional research is still needed as the confidence intervals are wide and the number of studies is small.
This analysis was carried out to compare the relative efficacy of these two drugs. Strictly speaking, we should have stated at the outset, whether we were testing for equivalence or superiority. By default, the method that we used was that used for superiority of one drug over another. Studies that set out to test equivalence require much larger patient numbers and a statement concerning the limits of equivalence. These are conventionally set at +/‐ 50% of the treatment effect for one of the drugs against an inactive control. These limits are rarely set, however, since they have not been established. In this context, we do have an estimate of efficacy, because in these studies a fall in FEV1 less than 10‐15% was judged to provide complete protection. Thus it might be reasonable to set limits of +/‐ 7.5 %. In only one study (Morton), was the mean effect outside these limits and the pooled effect size lay well within them.
As with any meta‐analysis, certain limitations of the review may exist, and the possibility of publication and selection biases should be addressed. A comprehensive search of published literature for potentially relevant studies was conducted, and attempts were made to contact corresponding and first authors to identify unpublished work. Often publication bias exists when negative trials, indicating no significant differences between drugs, are not published so not included in a review. Considering that the results of all the trials in this review independently illustrated no significant difference between the two medications, the identification of further negative trials should not alter this conclusion. Though it is possible that a selection bias occurred, we employed two independent reviewers for the selection process, and we are confident that the studies excluded were done so for appropriate reasons and in a consistent manner.
Authors' conclusions
Implications for practice.
Nedocromil and cromoglycate are equally effective in reducing or preventing exercise induced broncho‐constriction for up to 2 hours post inhalation. Beyond this time, neither drug offers significant therapeutic value. All doses of both drugs appear to be equally effective, so there is no advantage to the use of higher doses. Nedocromil seems to cause more bad taste. All participants in the studies had stable asthma, and experienced a wide range of severity of EIB on control and placebo runs, so the results of this review may be generalized to all stable asthmatics with exercise‐induced bronchoconstriction.
Implications for research.
Further research should focus on comparing these agents to other EIB medications (such as salbutamol, leukotriene modifier, ICS, and long acting bronchodilators.).
What's new
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 1 December 2007 | New search has been performed | One study met the eligibility criteria of the review. This study reported as a conference abstract did not report data that we could use in the meta‐analysis of our review. |
| 28 March 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors wish to acknowledge the assistance of Stephen Milan, Anna Bara and Jane Dennis of the Cochrane Airways Review Group. We would also like to acknowledge the assistance of the following corresponding authors: Dr. F. de Benedictis, Sinclair and Comis. Finally, the assistance of the reviewer and Professor Paul Jones (ARG Editor) in the reviewing the manuscript was greatly appreciated.
Data and analyses
Comparison 1. Nedocromil Sodium vs Cromolyn Sodium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum percent decrease in FEV‐1 | 7 | 194 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐4.49, 2.74] |
| 1.1 children | 5 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐4.38, 4.01] |
| 1.2 adults/combined | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐2.96 [‐13.21, 7.29] |
| 2 Failure to achieve Complete Protection: Maximum % fall >10% | 6 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.50, 1.81] |
| 2.1 Children | 4 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.39, 1.88] |
| 2.2 Adults/combination | 2 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.38, 3.63] |
| 3 Failure to achieve Complete Protection: Maximum % fall >15% | 6 | 170 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.33, 1.47] |
| 3.1 Children | 5 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.44, 1.81] |
| 3.2 Adults/combination | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.2 [0.04, 0.91] |
| 4 Failure to achieve Clinical Protection: <50% improvement over placebo | 6 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.39] |
| 4.1 Children | 4 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.79] |
| 4.2 Adults/combination | 2 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.22, 1.88] |
| 5 Side Effects | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 unpleasant taste | 3 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 6.85 [0.77, 60.73] |
| 5.2 sore throat | 2 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 3.46 [0.32, 37.47] |
1.1. Analysis.
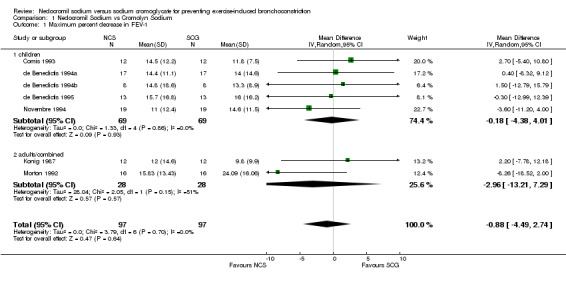
Comparison 1 Nedocromil Sodium vs Cromolyn Sodium, Outcome 1 Maximum percent decrease in FEV‐1.
1.2. Analysis.
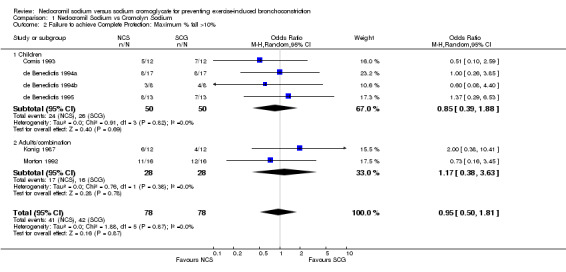
Comparison 1 Nedocromil Sodium vs Cromolyn Sodium, Outcome 2 Failure to achieve Complete Protection: Maximum % fall >10%.
1.3. Analysis.
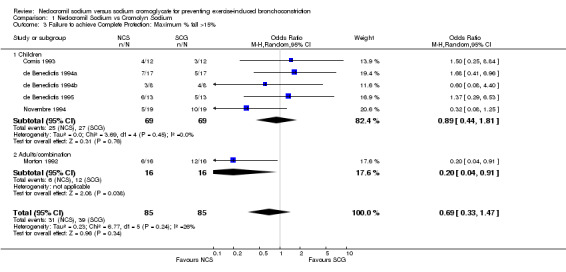
Comparison 1 Nedocromil Sodium vs Cromolyn Sodium, Outcome 3 Failure to achieve Complete Protection: Maximum % fall >15%.
1.4. Analysis.
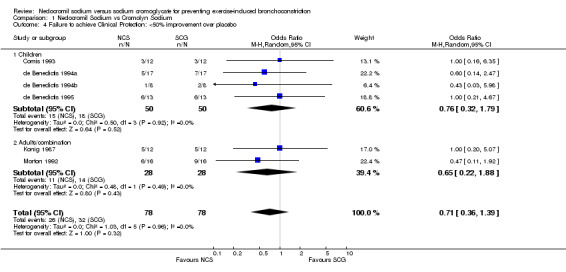
Comparison 1 Nedocromil Sodium vs Cromolyn Sodium, Outcome 4 Failure to achieve Clinical Protection: <50% improvement over placebo.
1.5. Analysis.
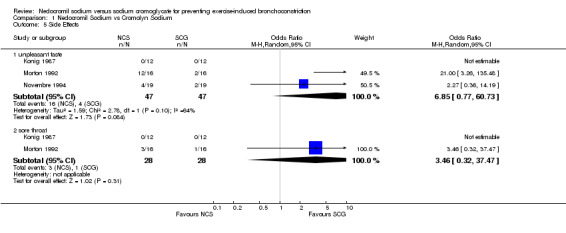
Comparison 1 Nedocromil Sodium vs Cromolyn Sodium, Outcome 5 Side Effects.
Comparison 2. Dosages.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum percent decrease in FEV‐1 | 7 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐4.49, 2.74] |
| 1.1 NCS 4 mg. vs SCG 10 mg. | 5 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐4.38, 4.01] |
| 1.2 NCS 4 mg. vs SCG 20 mg. | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐7.78, 12.18] |
| 1.3 NCS 8 mg. vs SCG 4 mg. | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐8.26 [‐18.52, 2.00] |
| 2 Failure to achieve Complete Protection: Maximum % fall >10% | 6 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.50, 1.81] |
| 2.1 NCS 4 mg. vs SCG 10 mg. | 4 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.39, 1.88] |
| 2.2 NCS 4 mg. vs SCG 20 mg. | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 2.0 [0.38, 10.41] |
| 2.3 NCS 8 mg. vs SCG 4 mg. | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.16, 3.45] |
| 3 Failure to achieve Complete Protection: Maximum % fall >15% | 6 | 170 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.33, 1.47] |
| 3.1 NCS 4 mg. vs SCG 10 mg. | 5 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.44, 1.81] |
| 3.2 NCS 4 mg. vs SCG 20 mg. | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 NCS 8 mg. vs SCG 4 mg. | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.2 [0.04, 0.91] |
| 4 Failure to achieve Clinical Protection: <50% improvement over placebo | 6 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.39] |
| 4.1 NCS 4 mg. vs SCG 10 mg. | 4 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.79] |
| 4.2 NCS 4 mg. vs SCG 20 mg. | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.20, 5.07] |
| 4.3 NCS 8 mg. vs SCG 4 mg. | 1 | 32 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.11, 1.92] |
2.1. Analysis.
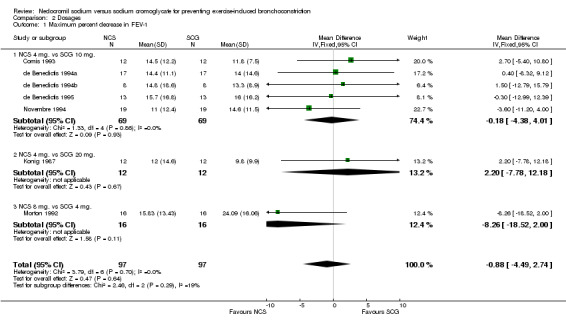
Comparison 2 Dosages, Outcome 1 Maximum percent decrease in FEV‐1.
2.2. Analysis.
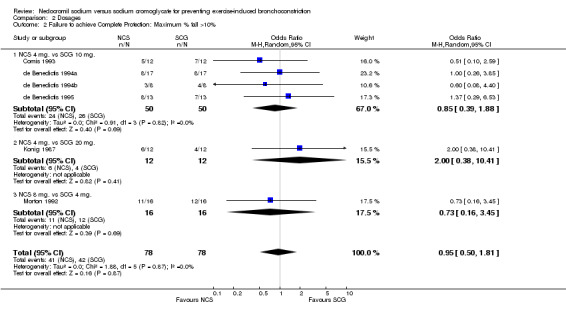
Comparison 2 Dosages, Outcome 2 Failure to achieve Complete Protection: Maximum % fall >10%.
2.3. Analysis.
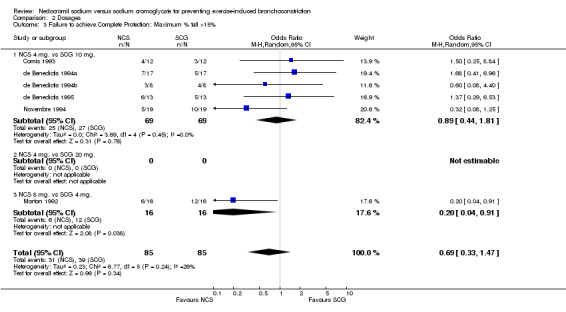
Comparison 2 Dosages, Outcome 3 Failure to achieve Complete Protection: Maximum % fall >15%.
2.4. Analysis.
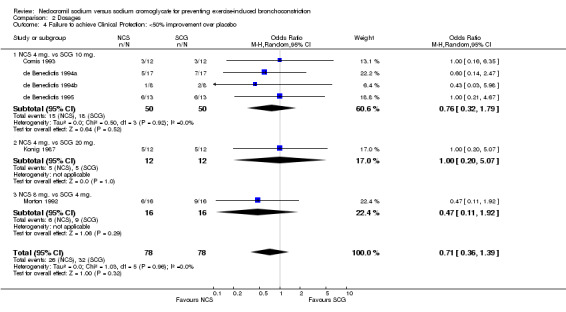
Comparison 2 Dosages, Outcome 4 Failure to achieve Clinical Protection: <50% improvement over placebo.
Comparison 3. Timing of Exercise ‐ post inhalation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum percent decrease in FEV‐1 | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 exercise <= 30 min. | 7 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐4.49, 2.74] |
| 1.2 exercise 120 ‐ 140 min. | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐3.01, 13.01] |
| 1.3 exercise 240 min. | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐10.42, 12.82] |
| 2 Failure to achieve Complete Protection: Maximum % fall >10% | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 exercise <= 30 min. | 6 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.50, 1.81] |
| 2.2 exercise 120 ‐ 140 min. | 2 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [0.52, 6.09] |
| 2.3 exercise 240 min. | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.22, 12.35] |
| 3 Failure to achieve Complete Protection: Maximum % fall >15% | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 exercise <= 30 min. | 6 | 170 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.35, 1.48] |
| 3.2 exercise 120 ‐ 140 min. | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [0.39, 8.89] |
| 3.3 exercise 240 min. | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Failure to achieve Clinical Protection: <50% improvement over placebo | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 exercise <= 30 min. | 6 | 156 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.39] |
| 4.2 exercise 120 ‐ 140 min. | 2 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 3.30 [0.83, 13.11] |
| 4.3 exercise 240 min. | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 2.5 [0.36, 17.32] |
3.1. Analysis.
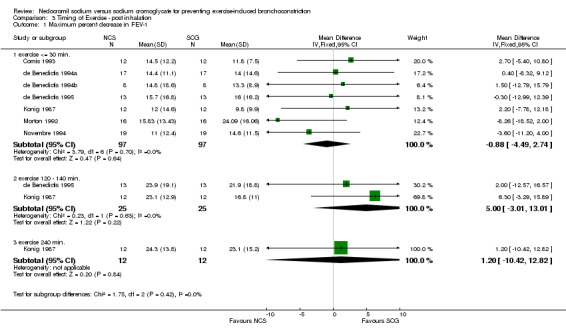
Comparison 3 Timing of Exercise ‐ post inhalation, Outcome 1 Maximum percent decrease in FEV‐1.
3.2. Analysis.
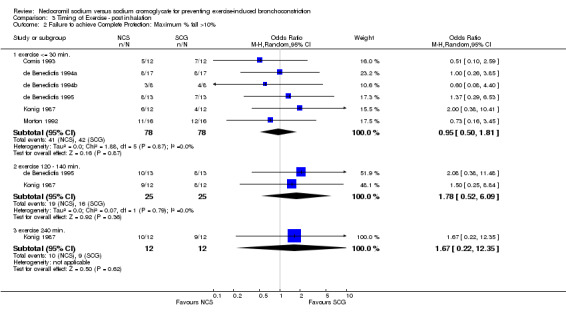
Comparison 3 Timing of Exercise ‐ post inhalation, Outcome 2 Failure to achieve Complete Protection: Maximum % fall >10%.
3.3. Analysis.
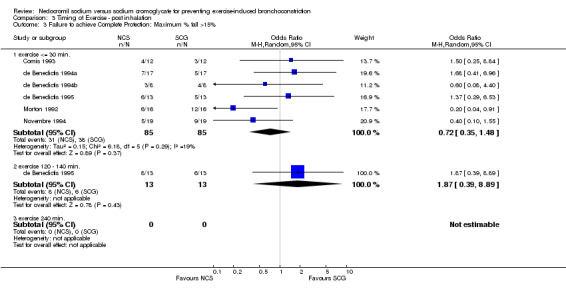
Comparison 3 Timing of Exercise ‐ post inhalation, Outcome 3 Failure to achieve Complete Protection: Maximum % fall >15%.
3.4. Analysis.
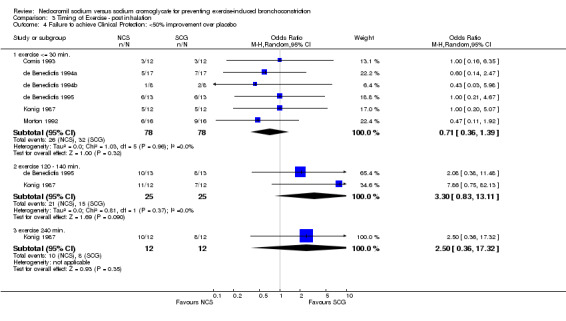
Comparison 3 Timing of Exercise ‐ post inhalation, Outcome 4 Failure to achieve Clinical Protection: <50% improvement over placebo.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cekic 2002.
| Methods | Design: randomized, single‐blind, placebo‐controlled crossover study x 4 study days. Method of randomization not described. Withdrawals: none | |
| Participants | Yugoslavia. Recruitment: unclear. N=45, gender not reported. Mean age 28 years. Inclusion: EIB, no other entry criterion described. Witheld: not described. | |
| Interventions | Randomised to: NCS, SCG, placebo via MDI 20 mins prior to exercise test. Exercise test: cycle ergometer | |
| Outcomes | Protection (given as number of participants who had protection against fall in FEV1, threshold not given) | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Comis 1993.
| Methods | Design: randomized, double‐blind, placebo‐controlled crossover study x 7 study days. Tests performed at same time daily (each patient completed within 10 days). Method of randomization not described. Withdrawals: none . | |
| Participants | Italy. Recruitment: residential school for asthmatics. N= 12: 7 m, 5 f. Age: 6.5 ‐ 13.5 (mean 11 yrs) Inclusion: Asthmatic (ATS def), atopic, history of EIA (fall in FEV1 > 15%) Witheld: Inhaled steroids, and SCG x 1 wk., B2 Agonists x 12 h. | |
| Interventions | Randomized to: NCS 4 mg, SCG 10 mg, or placebo via MDI alone or with a 700 ml spacer 30 min. pre test. Placebo = propellant only. Inhalation technique supervised. Exercise test: inclined treadmill, 6 min. Pulse = 180. | |
| Outcomes | Used a Vitalograph compact spirometer. Measured FEV1 before Tx, 30 min after Tx and at end exercise, then 1, 6, 11, 16, 21, 26, 30 min. post test. Calculated max % fall in FEV1 (labelled as % fall in FEV1), % protection. Side effects: not mentioned. Outcomes separately for MDI and Spacer. | |
| Notes | Jadad score = 3 Author confirmed data extraction within limits, could not access old data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
de Benedictis 1994a.
| Methods | Design: randomized, double‐blind, placebo‐controlled crossover study. 3 tests at same time on separate days, completed within 10 days. Method of randomization not clearly described. Withdrawals: not mentioned. | |
| Participants | Italy. Recruitment: pediatric asthma clinic. N=17: 11 m, 6 f. Age: 7‐15 mean 10.2 +/‐ 2.2 yr. Inclusion: asthma (ATS criteria), reproducible EIA (fall in FEV1 > 15%). Baseline FEV1 must be > 70% predicted normal and vary < 10% from previous study day. Excluded: patients with URI in previous 4 wks, no patients were taking oral steroids. Witheld: sustained‐release theophylline x 24 hr., all drugs x 12 hr. | |
| Interventions | Random, blinded order: NCS 4 mg or SCG 10 mg or placebo via MDI (2 used a spacer) using closed lip technique 20 min. pre exercise test. Exercise test: inclined treadmill x 6 min. HR = 85%. | |
| Outcomes | Used: turbine spirometer, & Knudson's predicted values. Measured: FEV1 pre Tx, pre‐exercise then 3, 5, 10, 15, 30 min. post exercise. Calculated: Max % fall in FEV1, % protection, complete protection (% decr. FEV1 < 10%), clinical protection. Time course estimated from graph. Side effects: not mentioned. | |
| Notes | Jadad score = 3 Author contacted and confirmed data extraction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
de Benedictis 1994b.
| Methods | Design: randomized, double‐blind, placebo‐controlled crossover study. 3 test days at same time on separate days, completed within 10 days. Method of randomization not clearly described. Withdrawals: not mentioned. | |
| Participants | Italy. Recruitment: pediatric asthma clinic. N=8 children, 5 m, 3 f. Age: 7‐11 (mean 8.7) Inclusion: asthmatic (ATS criteria), reproducible EIA (fall in FEV1 > 15%), baseline FEV1 must be > 70% predicted normal and vary < 10% from previous study day. Excluded: patients with URI in past 4 wks, no patients on oral steroids. Witheld: sustained‐release theophylline x 24 hr. other drugs x 12 hr. | |
| Interventions | Randomized order to: NCS 4 mg or SCG 10 mg or placebo via MDI with Aerochamber spacer 20 min. pre‐exercise test. Inhalation technique monitored. Exercise test: inclined treadmill, 6 min., pulse=85% of max predicted for age. | |
| Outcomes | Used turbine spirometer & Knudson's predicted values. Measured: FEV1 pre Tx, pre‐exercise then 3, 5, 10, 15, 30 min. post exercise. Calculated: Max % fall in FEV1, % protection, complete protection (% decr. FEV1<10%), clinical protection. Side effects: not mentioned. | |
| Notes | Jadad score = 3 Author contacted and confirmed data extraction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
de Benedictis 1995.
| Methods | Design: randomized, double‐blind, placebo‐controlled, crossover study. 3 test days at same time each day, completed within 10 days. Method of randomization not clearly described. Withdrawals: not mentioned. | |
| Participants | Italy. Recruitment: pediatric asthma clinic. N=13: 9 m, 4 f. Age: 7‐15 (mean 10 sd 2.3) Inclusion: asthmatic (ATS criteria), reproducible EIA (fall in FEV1 at least 15%), baseline FEV1 must be > 70% predicted normal and varied < 10% from previous study day. Excluded: No URI in previous 4 wks, no patients on oral steroids. Witheld: sustained‐release theophylline x 24 hr, other drugs x 12 hrs. | |
| Interventions | Random, blinded order: NCS 4 mg or SCG 10 mg or placebo via MDI using closed lip technique 20 min. & 140 min. pre exercise test. Technique monitored. Exercise test: inclined treadmill, 6 min., pulse=85% of max predicted for age. | |
| Outcomes | Used: turbine spirometer & Knudson's predicted values. Measured: FEV1 pre Tx, pre‐exercise then 3, 5, 10, 15, 30 min. post exercise. Calculated: Max % fall in FEV1, % protection., complete protection (% dec. FEV1<10%), clinical protection. Side effects: not mentioned. | |
| Notes | Jadad score = 3 Author stated that subjects in these three studies were descrete individuals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Konig 1987.
| Methods | Design: randomized, double blind, double‐dummy, placebo‐controlled, crossover study. 3 test days at same time of day, completed in 10 days. Participant performed 3 exercise tests on each study day. Method of randomization not described. Withdrawals: not mentioned. | |
| Participants | USA. N = 12 m. Age: 21‐38 (mean 27.3) Inclusion: asthmatic (ATS criteria), reproducible EIA (fall in FEV1 at least 20%), baseline FEV1 > 70% normal, no URI's in last 3 wks. Excluded: if on SCG or oral steroids in last month. FEV1 varied <15% between test days. Witheld: sustained‐release theophylline x 48 hrs, bronchodilators x 12 hrs. | |
| Interventions | In random order: 1) NCS 4 mg MDI plus placebo spinhaler capsule. 2) placebo MDI plus SCG 20 mg spinhaler capsule or 3) placebo MDI plus placebo spinhaler capsule 20 min. pre exercise test. Technique monitored. Test repeated at 2 & 4 hrs. post Tx. Exercise test: Inclined treadmill, 6 min., HR = 90% max predicted for age. | |
| Outcomes | Used a wedge spirometer & Knudson predicted values. Measured: FEV1, FVC, FEF 25‐75. pre Tx, 20 min. post Tx, then 3, 5, 10, 15, 20, 30 min. post exercise. Exercise test repeated without additional medication at 120 & 240 min. post Tx. Calculated: Max % fall FEV1, % protection, complete protection (% dec FEV1 <10%), clinical protection. Side effects: none from either drug. Statistics: Anova | |
| Notes | Jadad score = 3 No author contact to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Morton 1992.
| Methods | Design: randomized, double‐blind, placebo‐controlled, crossover study. 4 study days at same time of day, completed in 11 days. Method of randomization not described. Withdrawals: described. | |
| Participants | Australia. N = 16, 10 m, 6 f. Age: 13‐30 (mean 20, sd 4.84) Inclusion: asthmatic (ATS criteria), non‐smokers, history of EIA ( at least a 15% fall in FEV1) FEV1 > 75% of baseline) 2 m & 1 f excluded on this basis ‐ 16 patients analyzed. Excluded if on oral steroids in last 4 wks. Witheld: short & long acting B agonists x 4 & 12 hrs. SCG, slow‐release methyl xanthines and H1 antagonists x 24 hrs. Must be on current ICS dose x 4 wks. No food or fluid 2 h. pre‐test, allowed only 1 cup of caffeinated fluid or 1 chocolate bar on test day, avoided vigorous exercise for 24 h and total abstinence from exercise for 4 h. pre‐test. | |
| Interventions | Random assignment to NCS 8 mg, SCG 4 mg, placebo (propellant gas & sorbitan trioleate) or no TX via identical MDI's 15 min. pre‐exercise test. Technique monitored. Exercise test: Inclined treadmill, 8 min, VO2 must be >= 70% max. | |
| Outcomes | Used a single wedge dry spirometer (highest of 2 trials). Measured: FEV1 pre‐Tx, pre‐exercise and immediately then 5, 10, 15, 20, 25 & 30 min. post exercise. Calculated: % of premed FEV1, max % dec FEV1, Complete protection (% dec FEV1 <15%) for mild, moderate and severe asthmatics. 2 trials performed and highest value recorded. Time course estimated from graph. Side effects: unpleasant taste, throat irritation. | |
| Notes | Jadad score = 4 No author contact to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Novembre 1994.
| Methods | Design: randomized, double‐blind, placebo‐controlled crossover study. 3 test days at same time of day. Method of randomization not described. Withdrawals: not mentioned. | |
| Participants | Italy. Recruitment: not described. N = 19. 13 m, 6 f. Age: 6‐15. Inclusion: asthmatic (all relieved with antiasthmatic drugs), atopic, history of EIA (Fall in FEV1 at least 15 %), Excluded: SCG or inhaled steroids in past month, no URI in past 3 wks, Witheld:bronchodilators and long acting inhaled B‐2 agonists x 12 hr, short acting inhaled bronchodilators x 6 hrs. Concommittant meds: All on inhaled B‐2 agonists, 6 on SCG, 3 on theophylline, 2 on beclomethasone. Baseline FEV1 > 92% predicted normal at time of testing. | |
| Interventions | In random order: NCS 4 mg or SCG 10 mg or placebo via MDI plus large volume spacer 20 min pre‐exercise test. Placebo= propellant only. Technique monitored. Exercise test: Inclined treadmill x 6 min., HR = 170‐180. | |
| Outcomes | Measured with pneumotachograph. Measured: FEV1, PEFR, FEF 25‐75 pre‐Tx, pre‐exercise test, then 1, 5, 10, 15, 20 min post ex. Calculated: max % fall FEV1, PEFR and FEF25‐75, mean % fall FEV1 at time points,% predicted normal, % protection, complete protection (% dec FEV1 <15%) Side effects: unpleasant taste. | |
| Notes | Jadad score = 3 No author contact to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Sinclair 1990.
| Methods | Design: randomized, double‐blind, placebo‐controlled, cross over study. Method of randomization not described. Tests on 3 successive days between 9:30 ‐ 11:30 AM. Withdrawals: not mentioned. | |
| Participants | UK. Recruitment: not described. N=20. 18 m, 2 f Age: mean=20.7; range=17‐28. Inclusion: reproducible EIA (EIA not defined). Excluded: patients taking inhaled or oral steroids, NCS or SCG. Witheld: bronchodilators x 24 hrs. | |
| Interventions | Randomized to: NCS 4 mg, SCG 10 mg or placebo via coded MDI's 30 min. pre exercise. Exercise test: inclined treadmill, 6 min, 10% incline. | |
| Outcomes | Used Vitalograph. Measured: FEV1 pre exercise then 1, 3, 5, 7, 9 min. post exercise. Calculated: mean % fall FEV1 at time points‐ values approximated from graph. | |
| Notes | Jadad score = 5 Author contacted. Confirmed >= 15% fall in FEV1 for diagnosis of EIB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Coded in pharmacy, code blind until end of study |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Debelic 1986 | Compared NSC and placebo. |
| deBenedictis 1998 | Compared salbutamol and salbutamol/nedocromil. |
| Hoffmeister 1995 | No comparison of NCS and SCG. |
| Magnussen 1986 | Not a RCT. |
| Shaw 1986 | No SCG group |
| Todaro 1993 | Compared NSC and placebo only. |
Contributions of authors
Karen Kelly: Project coordinator, initial searches & screening, study selection, quality assessments, form design, author correspondence, statistics, data extraction & entry, primary author. Carol Spooner: study selection, quality assessments, data extraction & entry, editorial review. Converted to RevMan4. BH Rowe: study selection, quality assessments, editorial review, ARG assigned editor.
Sources of support
Internal sources
Division of Emergency Medicine, University of Alberta, Faculty of Medicine and Dentistry, Edmonton, AB, Canada.
NHS Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
The authors who are involved in this review have done so without any known conflict of interest. They are neither involved with the primary studies nor affiliated with any pharmaceutical company that produces NCS or SCG.
Edited (no change to conclusions)
References
References to studies included in this review
Cekic 2002 {published data only}
- Cekic SS, Filipovic MD, Dimitrijevic OB. Comparison of salbutamol, nedocromil sodium and disodium cromoglycate (DSCG) protective effects in patients with exercise induced bronchoconstriction (EIB) [Abstract]. European Respiratory Journal 2002;20(Suppl 38):306s. [Google Scholar]
Comis 1993 {published data only}
- Comis A, Valletta EA, Sette L, Andreoli A, Boner AL. Comparison of nedocromil sodium and sodium cromoglycate administered by pressurized aerosol, with and without a spacer device in exercise‐ induced asthma in children. European Respiratory Journal 1993;6:523‐6. [PubMed] [Google Scholar]
de Benedictis 1994a {published data only}
- Benedictis FM, Tuteri G, Bertotto A, Bruni L, Vaccaro R. Comparison of the protective effects of cromolyn sodium and nedocromil sodium in the treatment of exercise‐induced asthma in children. Journal of Allergy & Clinical Immunology 1994;94:684‐8. [DOI] [PubMed] [Google Scholar]
de Benedictis 1994b {published data only}
- Benedictis FM, Tuteri G, Niccoli A, Mezzetti D, Rossi L, Bruni L. The effect of cromolyn sodium and nedocromil sodium administered by a pressurized aerosol with a spacer device on exercise‐induced asthma in children. Mediators of Inflammation 1994;3(S1):S35‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Benedictis 1995 {published data only}
- Benedictis FM, Tuteri G, Pazzelli P, Bertotto A, Bruni L, Vaccaro R. Cromolyn versus nedocromil: Duration of action in exercise‐induced asthma in children. Journal of Allergy & Clinical Immunology 1995;96:510‐4. [DOI] [PubMed] [Google Scholar]
Konig 1987 {published data only}
- Konig P, Hordvik NL, Kreutz C. The preventive effect and duration of action of nedocromil sodium and cromolyn sodium on exercise‐induced asthma (EIA) in adults. Journal of Allergy & Clinical Immunology 1987;79:64‐8. [DOI] [PubMed] [Google Scholar]
Morton 1992 {published data only}
- Morton AR, Ogle SL, Fitch KD. Effects of nedocromil sodium, cromolyn sodium, and a placebo in exercise‐induced asthma. Annals of Allergy 1992;68:143‐8. [PubMed] [Google Scholar]
Novembre 1994 {published data only}
- Novembre E, Frongia GF, Veneruso G, Vierucci A. Inhibition of exercise‐induced‐asthma (EIA) by nedocromil sodium and sodium cromoglycate in children. Pediatric Allergy and Immunology 1994;5:107‐10. [DOI] [PubMed] [Google Scholar]
Sinclair 1990 {published data only}
- Sinclair DG, Winfield CR. Attenuation of exercise induced asthma by nedocromil sodium and sodium cromoglycate. Journal of the Royal Army Medical Corps 1990;136:105‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Debelic 1986 {published data only}
- Debelic M. Nedocromil sodium and exercise‐induced asthma in adolescents. European Journal of Respiratory Diseases 1986;69 Suppl(147):266‐7. [Google Scholar]
deBenedictis 1998 {published data only}
- Benedictis FM, Tuteri G, Pazzelli P, Solinas LF, Noccoli A, Parente C. Combination drug therapy for the prevention of exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma, & Immunology 1998;80:352‐6. [DOI] [PubMed] [Google Scholar]
Hoffmeister 1995 {published data only}
- Hoffmeister BC, Casanova ZD. Sodium nedocromil and sodium cromoglycate in the prevention of exercise induced asthma [Nedocromil sodico y cromoglicato de sodio en la prevencion del aska inducida por ejercicio]. Revista Chilena de Pediatria 1995;66:296‐9. [Google Scholar]
Magnussen 1986 {published data only}
- Magnussen H. The protective effect of disodium cromoglycate and nedocromil sodium on exercise‐induced bronchial asthma. Atemwegs‐und Lungenkrankheiten 1986;12:S107‐9. [Google Scholar]
Shaw 1986 {published data only}
- Shaw RJ, Kay AB. Nedocromil sodium, a mucosal and connective tissue mast cell stabilizer, inhibits exercise‐induced asthma. European Journal of Respiratory Diseases 1986;69(Suppl 147):294‐6. [DOI] [PubMed] [Google Scholar]
Todaro 1993 {published data only}
- Todaro A, Faina M, Alippi B, Dal Monte A, Ruggieri F. Nedocromil sodium in the prevention of exercise‐induced bronchospasm in athletes with asthma. Journal of Sports Medicine and Physical Fitness 1993;33:137‐45. [PubMed] [Google Scholar]
Additional references
Anderson 1985
- Anderson SD. Issues in exercise‐induced asthma. Journal of Allergy & Clinical Immunology 1985;76:763‐72. [DOI] [PubMed] [Google Scholar]
Anderson 1995
- Anderson SD. Specific problems: exercise‐induced asthma. In: O'Byrne P, Thomson NC editor(s). Manual of Asthma Management. London: WB Saunders Co. Ltd, 1995:621‐43. [Google Scholar]
Anon 1999
- Anon. Drugs for Asthma. Medical Letter on Drugs and Therapeutics 1999;41(1044):5‐10. [PubMed] [Google Scholar]
Bar‐Yishay 1984
- Bar‐Yishay E, Godfrey S. Mechanisms of exercise‐induced asthma. Lung 1984;162(195):851‐5. [DOI] [PubMed] [Google Scholar]
CPS 1999
- CPS. Compendium of Pharmaceuticals and Specialties. 34. Ottawa: Canadian Pharmaceutical Association, 1999. [Google Scholar]
Eggleston 1984
- Eggleston PA. Methods of exercise challenge. Journal of Allergy & Clinical Immunology 1984;73:666‐9. [DOI] [PubMed] [Google Scholar]
Holgate 1986
- Holgate ST. Clinical evaluation of nedocromil sodium in asthma. European Journal of Respiratory Diseases 1986;69(Suppl 147):149‐59. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Rupp 1996
- Rupp NT. Diagnosis and management of exercise‐induced asthma. Physician & Sportsmedicine 1996;24(1):77‐7. [DOI] [PubMed] [Google Scholar]
Selcow 1989
- Selcow JE, Mendelson LM, Rosen JP. Clinical benefits of cromolyn sodium aerosol (MDI) in the treatment of asthma in children. Annals of Allergy 1989;62:195‐9. [PubMed] [Google Scholar]
Sly 1984
- Sly RM. Beta‐adrenergic drugs in the management of asthma in athletes. Journal of Allergy & Clinical Immunology 1984;73:680‐5. [DOI] [PubMed] [Google Scholar]
Spector 1993
- Spector SL. Update on exercise‐induced asthma. Annals of Allergy 1993;71:571‐7. [PubMed] [Google Scholar]
Spooner 2002
- Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise‐induced bronchoconstriction (Cochrane Review). Cochrane Database of Systematic Reviews 2002, Issue 1. [Art. No.: CD001183. DOI: 10.1002/14651858.CD001183.] [DOI] [PubMed] [Google Scholar]
Virant 1992
- Virant FS. Exercise‐induced bronchospasm: epidemiology, pathophysiology, and therapy. Medicine & Science in Sports & Exercise 1992;24:851‐5. [PubMed] [Google Scholar]


